Genetic determinants of circulating sphingolipid concentrations in European populations
- PMID: 19798445
- PMCID: PMC2745562
- DOI: 10.1371/journal.pgen.1000672
Genetic determinants of circulating sphingolipid concentrations in European populations
Abstract
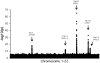
Sphingolipids have essential roles as structural components of cell membranes and in cell signalling, and disruption of their metabolism causes several diseases, with diverse neurological, psychiatric, and metabolic consequences. Increasingly, variants within a few of the genes that encode enzymes involved in sphingolipid metabolism are being associated with complex disease phenotypes. Direct experimental evidence supports a role of specific sphingolipid species in several common complex chronic disease processes including atherosclerotic plaque formation, myocardial infarction (MI), cardiomyopathy, pancreatic beta-cell failure, insulin resistance, and type 2 diabetes mellitus. Therefore, sphingolipids represent novel and important intermediate phenotypes for genetic analysis, yet little is known about the major genetic variants that influence their circulating levels in the general population. We performed a genome-wide association study (GWAS) between 318,237 single-nucleotide polymorphisms (SNPs) and levels of circulating sphingomyelin (SM), dihydrosphingomyelin (Dih-SM), ceramide (Cer), and glucosylceramide (GluCer) single lipid species (33 traits); and 43 matched metabolite ratios measured in 4,400 subjects from five diverse European populations. Associated variants (32) in five genomic regions were identified with genome-wide significant corrected p-values ranging down to 9.08x10(-66). The strongest associations were observed in or near 7 genes functionally involved in ceramide biosynthesis and trafficking: SPTLC3, LASS4, SGPP1, ATP10D, and FADS1-3. Variants in 3 loci (ATP10D, FADS3, and SPTLC3) associate with MI in a series of three German MI studies. An additional 70 variants across 23 candidate genes involved in sphingolipid-metabolizing pathways also demonstrate association (p = 10(-4) or less). Circulating concentrations of several key components in sphingolipid metabolism are thus under strong genetic control, and variants in these loci can be tested for a role in the development of common cardiovascular, metabolic, neurological, and psychiatric diseases.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Zheng W, Kollmeyer J, Symolon H, Momin A, Munter E, et al. Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta. 2006;1758:1864–1884. - PubMed
-
- Kolter T, Sandhoff K. Sphingolipid metabolism diseases. Biochim Biophys Acta. 2006;1758:2057–2079. - PubMed
-
- Dawkins JL, Hulme DJ, Brahmbhatt SB, Auer-Grumbach M, Nicholson GA. Mutations in SPTLC1, encoding serine palmitoyltransferase, long chain base subunit-1, cause hereditary sensory neuropathy type I. Nat Genet. 2001;27:309–312. - PubMed
-
- Simpson MA, Cross H, Proukakis C, Priestman DA, Neville DC, et al. Infantile-onset symptomatic epilepsy syndrome caused by a homozygous loss-of-function mutation of GM3 synthase. Nat Genet. 2004;36:1225–1229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

