The role of osteopontin in inflammatory processes
- PMID: 19798593
- PMCID: PMC2778587
- DOI: 10.1007/s12079-009-0068-0
The role of osteopontin in inflammatory processes
Abstract
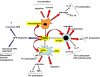
Osteopontin (OPN) is a matricellular protein that mediates diverse biological functions. OPN is involved in normal physiological processes and is implicated in the pathogenesis of a variety of disease states, including atherosclerosis, glomerulonephritis, cancer, and several chronic inflammatory diseases. Through interactions with several integrins, OPN mediates cell migration, adhesion, and survival in many cell types. OPN also functions as a Th1 cytokine, promotes cell-mediated immune responses, and plays a role in chronic inflammatory and autoimmune diseases. Besides its function in inflammation, OPN is also a regulator of biomineralization and a potent inhibitor of vascular calcification.
Figures

References
-
- Agnholt J, Kelsen J, Schack L, Hvas CL, Dahlerup JF, Sorensen ES. Osteopontin, a protein with cytokine-like properties, is associated with inflammation in Crohn’s disease. Scand J Immunol. 2007;65(5):453–460. - PubMed
-
- Agnihotri R, Crawford HC, Haro H, Matrisian LM, Havrda MC, Liaw L. Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin) J Biol Chem. 2001;276(30):28261–28267. - PubMed
-
- Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, Rittling SR, Denhardt DT, Glimcher MJ, Cantor H. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287(5454):860–864. - PubMed
-
- Bayless KJ, Davis GE. Identification of dual alpha4 beta1 integrin binding sites within a 38 amino acid domain in the N-terminal thrombin fragment of human osteopontin. J Biol Chem. 2001;276(16):13483–13489. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

