Hybrid quantum/classical molecular dynamics simulations of the proton transfer reactions catalyzed by ketosteroid isomerase: analysis of hydrogen bonding, conformational motions, and electrostatics
- PMID: 19799395
- PMCID: PMC2783618
- DOI: 10.1021/bi901353v
Hybrid quantum/classical molecular dynamics simulations of the proton transfer reactions catalyzed by ketosteroid isomerase: analysis of hydrogen bonding, conformational motions, and electrostatics
Abstract
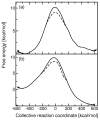
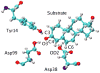
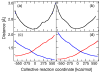
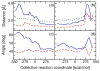
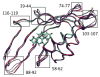
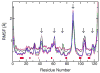
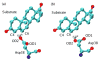
Hybrid quantum/classical molecular dynamics simulations of the two proton transfer reactions catalyzed by ketosteroid isomerase are presented. The potential energy surfaces for the proton transfer reactions are described with the empirical valence bond method. Nuclear quantum effects of the transferring hydrogen increase the rates by a factor of approximately 8, and dynamical barrier recrossings decrease the rates by a factor of 3-4. For both proton transfer reactions, the donor-acceptor distance decreases substantially at the transition state. The carboxylate group of the Asp38 side chain, which serves as the proton acceptor and donor in the first and second steps, respectively, rotates significantly between the two proton transfer reactions. The hydrogen-bonding interactions within the active site are consistent with the hydrogen bonding of both Asp99 and Tyr14 to the substrate. The simulations suggest that a hydrogen bond between Asp99 and the substrate is present from the beginning of the first proton transfer step, whereas the hydrogen bond between Tyr14 and the substrate is virtually absent in the first part of this step but forms nearly concurrently with the formation of the transition state. Both hydrogen bonds are present throughout the second proton transfer step until partial dissociation of the product. The hydrogen bond between Tyr14 and Tyr55 is present throughout both proton transfer steps. The active site residues are more mobile during the first step than during the second step. The van der Waals interaction energy between the substrate and the enzyme remains virtually constant along the reaction pathway, but the electrostatic interaction energy is significantly stronger for the dienolate intermediate than for the reactant and product. Mobile loop regions distal to the active site exhibit significant structural rearrangements and, in some cases, qualitative changes in the electrostatic potential during the catalytic reaction. These results suggest that relatively small conformational changes of the enzyme active site and substrate strengthen the hydrogen bonds that stabilize the intermediate, thereby facilitating the proton transfer reactions. Moreover, the conformational and electrostatic changes associated with these reactions are not limited to the active site but rather extend throughout the entire enzyme.
Figures


Similar articles
-
Catalytic efficiency of enzymes: a theoretical analysis.Biochemistry. 2013 Mar 26;52(12):2012-20. doi: 10.1021/bi301515j. Epub 2012 Dec 20. Biochemistry. 2013. PMID: 23240765 Free PMC article. Review.
-
Impact of mutation on proton transfer reactions in ketosteroid isomerase: insights from molecular dynamics simulations.J Am Chem Soc. 2010 Jun 2;132(21):7549-55. doi: 10.1021/ja102714u. J Am Chem Soc. 2010. PMID: 20450180 Free PMC article.
-
Contribution of the hydrogen-bond network involving a tyrosine triad in the active site to the structure and function of a highly proficient ketosteroid isomerase from Pseudomonas putida biotype B.Biochemistry. 2000 Apr 25;39(16):4581-9. doi: 10.1021/bi992119u. Biochemistry. 2000. PMID: 10769113
-
Computational study of ketosteroid isomerase: insights from molecular dynamics simulation of enzyme bound substrate and intermediate.J Am Chem Soc. 2003 Jun 25;125(25):7553-61. doi: 10.1021/ja030138s. J Am Chem Soc. 2003. PMID: 12812495
-
Structure and enzymology of Delta5-3-ketosteroid isomerase.Curr Opin Struct Biol. 2001 Dec;11(6):674-8. doi: 10.1016/s0959-440x(01)00268-8. Curr Opin Struct Biol. 2001. PMID: 11751047 Review.
Cited by
-
Water in the active site of ketosteroid isomerase.Biochemistry. 2011 Aug 9;50(31):6689-700. doi: 10.1021/bi200703y. Epub 2011 Jul 13. Biochemistry. 2011. PMID: 21710970 Free PMC article.
-
Catalytic efficiency of enzymes: a theoretical analysis.Biochemistry. 2013 Mar 26;52(12):2012-20. doi: 10.1021/bi301515j. Epub 2012 Dec 20. Biochemistry. 2013. PMID: 23240765 Free PMC article. Review.
-
Using unnatural amino acids to probe the energetics of oxyanion hole hydrogen bonds in the ketosteroid isomerase active site.J Am Chem Soc. 2014 May 28;136(21):7643-54. doi: 10.1021/ja413174b. Epub 2014 May 14. J Am Chem Soc. 2014. PMID: 24787954 Free PMC article.
-
From alcohol dehydrogenase to a "one-way" carbonyl reductase by active-site redesign: a mechanistic study of mannitol 2-dehydrogenase from pseudomonas fluorescens.J Biol Chem. 2010 Oct 1;285(40):30644-53. doi: 10.1074/jbc.M110.108993. Epub 2010 Jul 16. J Biol Chem. 2010. PMID: 20639204 Free PMC article.
-
Experimental and computational mutagenesis to investigate the positioning of a general base within an enzyme active site.Biochemistry. 2014 Apr 22;53(15):2541-55. doi: 10.1021/bi401671t. Epub 2014 Apr 9. Biochemistry. 2014. PMID: 24597914 Free PMC article.
References
-
- Pollack RM. Enzymatic mechanisms for catalysis of enolization: ketosteroid isomerase. Bioorg Chem. 2004;32:341–353. - PubMed
-
- Hawkinson DC, Eames TCM, Pollack RM. Energetics of 3-oxo-Δ5-steroid isomerase: Source of the catalytic power of the enzyme. Biochemistry. 1991;30:10849–10858. - PubMed
-
- Wu ZR, Ebrahimian S, Zawrotny ME, Thornburg LD, Perez-Alvarado GC, Brothers P, Pollack RM, Summers MF. Solution structure of 3-oxo-Δ5-steroid isomerase. Science. 1997;276:415–418. - PubMed
-
- Choi G, Ha N-C, Kim SW, Kim D-H, Park S, Oh B-H, Choi KY. Asp-99 donates a hydrogen bond not to Tyr-14 but to the steroid directly in the catalytic mechanism of Δ5-3-ketosteroid isomerase from Pseudomonas putida Biotype B. Biochemistry. 2000;39:903–909. - PubMed
-
- Brothers PN, Blotny G, Qi L, Pollack RM. An active site phenylalanine of 3-oxo-Δ5-steroid isomerase is catalytically important for proton transfer. Biochemistry. 1995;34:15453–15458. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

