Aberrant human leucocyte antigen-G expression and its clinical relevance in hepatocellular carcinoma
- PMID: 19799650
- PMCID: PMC3823007
- DOI: 10.1111/j.1582-4934.2009.00917.x
Aberrant human leucocyte antigen-G expression and its clinical relevance in hepatocellular carcinoma
Abstract
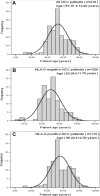
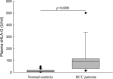
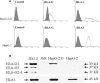
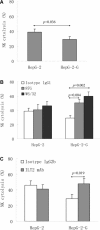
The clinical relevance of human leucocyte antigen-G (HLA-G) has been postulated in malignancies. Hepatocellular carcinoma (HCC) is a major contributor to cancer incidence and mortality worldwide; however, potential roles of HLA-G in HCC remain unknown. In the current study, HLA-G expression in 219 primary HCC lesions and their adjacent non-tumourous samples was analysed with immunohistochemistry. Correlations among HLA-G expression and various clinical parameters were evaluated. Meanwhile, functional analysis of transfected cell surface HLA-G expression on NK cell cytolysis was performed in vitro. HLA-G expression was observed in 50.2% (110/219) of primary HCC lesions, and undetectable in corresponding adjacent normal liver tissues. HLA-G expression was found in 37.8%, 41.9% and 71.4% of stage I, II and III HCC lesions, respectively. Data revealed that HLA-G expression in HCC was strongly correlated to advanced disease stage (I versus II, P= 0.882; I versus III, P= 0.020; II versus III, P= 0.037). HLA-G expression was also more frequently observed in elder patients (≥median 52 years, 57.5%versus 43.4%, P= 0.004). Meanwhile, plasma soluble HLA-G in HCC patients was significantly higher than that in normal controls (median, 92.49U/ml versus 9.29U/ml, P= 0.000). Functional assay showed that HLA-G expression in transfected cells could dramatically decrease the NK cell cytolysis (P= 0.036), which could be markedly restored by the blockade of HLA-G (P= 0.004) and its receptor ILT2 (P= 0.019). Our finding indicated that HLA-G expression was strongly correlated to advanced disease stage, and more frequently observed in elder patients. Its relevance to HCC progression might be result from the inhibition of NK cell cytolysis.
© 2009 The Authors Journal compilation © 2010 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures

Similar articles
-
Clinical relevance and functional implications for human leucocyte antigen-g expression in non-small-cell lung cancer.J Cell Mol Med. 2010 Sep;14(9):2318-29. doi: 10.1111/j.1582-4934.2009.00858.x. J Cell Mol Med. 2010. PMID: 19602033 Free PMC article.
-
The alpha1 domain of HLA-G1 and HLA-G2 inhibits cytotoxicity induced by natural killer cells: is HLA-G the public ligand for natural killer cell inhibitory receptors?Proc Natl Acad Sci U S A. 1997 May 13;94(10):5249-54. doi: 10.1073/pnas.94.10.5249. Proc Natl Acad Sci U S A. 1997. PMID: 9144223 Free PMC article.
-
Human leukocyte antigen-G protein expression is an unfavorable prognostic predictor of hepatocellular carcinoma following curative resection.Clin Cancer Res. 2009 Jul 15;15(14):4686-93. doi: 10.1158/1078-0432.CCR-09-0463. Epub 2009 Jul 7. Clin Cancer Res. 2009. PMID: 19584149
-
HLA-G promotes immune tolerance.J Biol Regul Homeost Agents. 2000 Apr-Jun;14(2):93-8. J Biol Regul Homeost Agents. 2000. PMID: 10841284 Review.
-
Human leucocyte antigen-G and reproduction.J Reprod Immunol. 1999 Jul;43(2):235-42. doi: 10.1016/s0165-0378(99)00023-6. J Reprod Immunol. 1999. PMID: 10479059 Review. No abstract available.
Cited by
-
The double-sided of human leukocyte antigen-G molecules in type 1 autoimmune hepatitis.Front Immunol. 2022 Oct 12;13:1007647. doi: 10.3389/fimmu.2022.1007647. eCollection 2022. Front Immunol. 2022. PMID: 36311782 Free PMC article.
-
Expression of human leukocyte antigen G is associated with prognosis in nasopharyngeal carcinoma.Int J Biol Sci. 2012;8(6):891-900. doi: 10.7150/ijbs.4383. Epub 2012 Jun 16. Int J Biol Sci. 2012. PMID: 22745579 Free PMC article.
-
Clinical Significance of Potential Unidentified HLA-G Isoforms Without α1 Domain but Containing Intron 4 in Colorectal Cancer Patients.Front Oncol. 2018 Sep 4;8:361. doi: 10.3389/fonc.2018.00361. eCollection 2018. Front Oncol. 2018. PMID: 30234020 Free PMC article.
-
Strategies for Overcoming Immune Evasion in Bladder Cancer.Int J Mol Sci. 2024 Mar 7;25(6):3105. doi: 10.3390/ijms25063105. Int J Mol Sci. 2024. PMID: 38542078 Free PMC article. Review.
-
Prognostic and Clinicopathological Value of Human Leukocyte Antigen G in Gastrointestinal Cancers: A Meta-Analysis.Front Oncol. 2021 May 12;11:642902. doi: 10.3389/fonc.2021.642902. eCollection 2021. Front Oncol. 2021. PMID: 34055611 Free PMC article.
References
-
- Ferrone S, Whiteside TL. Tumor microenvironment and immune escape. Surg Oncol Clin N Am. 2007;16:755–74. - PubMed
-
- Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27:5932–43. - PubMed
-
- Urosevic M, Dummer R. Human leukocyte antigen-G and cancer immunoediting. Cancer Res. 2008;68:627–30. - PubMed
-
- Polakova K, Bandzuchova E, Tirpakova J, et al. Modulation of HLA-G expression. Neoplasma. 2007;54:455–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

