ATF4-dependent transcription mediates signaling of amino acid limitation
- PMID: 19800252
- PMCID: PMC3587693
- DOI: 10.1016/j.tem.2009.05.008
ATF4-dependent transcription mediates signaling of amino acid limitation
Abstract
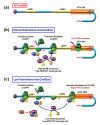
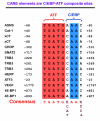
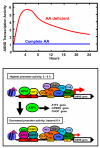
Mammals respond to dietary nutrient fluctuations; for example, deficiency of dietary protein or an imbalance of essential amino acids activates an amino acid response (AAR) signal transduction pathway, consisting of detection of uncharged tRNA by the GCN2 kinase, eIF2alpha phosphorylation and ATF4 expression. In concert with heterodimerization partners, ATF4 activates specific genes via a CCAAT-enhancer binding protein-activating transcription factor response element (CARE). This review outlines the ATF4-dependent transcriptional mechanisms associated with the AAR, focusing on progress during the past 5 years. Recent evidence suggests that maternal nutrient deprivation not only has immediate metabolic effects on the fetus, but also triggers gene expression changes in adulthood, possibly through epigenetic mechanisms. Therefore, understanding the transcriptional programs initiated by amino acid limitation is crucial and timely.
Figures

References
-
- Zhou D, et al. Phosphorylation of eIF2 Directs ATF5 Translational Control in Response to Diverse Stress Conditions. J. Biol. Chem. 2008;283:7064–7073. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

