Nitric oxide signaling in Pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic di-GMP levels, and enhanced dispersal
- PMID: 19801410
- PMCID: PMC2786556
- DOI: 10.1128/JB.00975-09
Nitric oxide signaling in Pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic di-GMP levels, and enhanced dispersal
Abstract
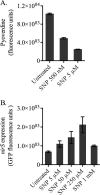
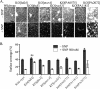
Bacteria in biofilms often undergo active dispersal events and revert to a free-swimming, planktonic state to complete the biofilm life cycle. The signaling molecule nitric oxide (NO) was previously found to trigger biofilm dispersal in the opportunistic pathogen Pseudomonas aeruginosa at low, nontoxic concentrations (N. Barraud, D. J. Hassett, S. H. Hwang, S. A. Rice, S. Kjelleberg, and J. S. Webb, J. Bacteriol. 188:7344-7353, 2006). NO was further shown to increase cell motility and susceptibility to antimicrobials. Recently, numerous studies revealed that increased degradation of the secondary messenger cyclic di-GMP (c-di-GMP) by specific phosphodiesterases (PDEs) triggers a planktonic mode of growth in eubacteria. In this study, the potential link between NO and c-di-GMP signaling was investigated by performing (i) PDE inhibitor studies, (ii) enzymatic assays to measure PDE activity, and (iii) direct quantification of intracellular c-di-GMP levels. The results suggest a role for c-di-GMP signaling in triggering the biofilm dispersal event induced by NO, as dispersal requires PDE activity and addition of NO stimulates PDE and induces the concomitant decrease in intracellular c-di-GMP levels in P. aeruginosa. Furthermore, gene expression studies indicated global responses to low, nontoxic levels of NO in P. aeruginosa biofilms, including upregulation of genes involved in motility and energy metabolism and downregulation of adhesins and virulence factors. Finally, site-directed mutagenesis of candidate genes and physiological characterization of the corresponding mutant strains uncovered that the chemotaxis transducer BdlA is involved in the biofilm dispersal response induced by NO.
Figures


Similar articles
-
Origin and Impact of Nitric Oxide in Pseudomonas aeruginosa Biofilms.J Bacteriol. 2016 Jan 1;198(1):55-65. doi: 10.1128/JB.00371-15. J Bacteriol. 2016. PMID: 26260455 Free PMC article. Review.
-
Induction of Native c-di-GMP Phosphodiesterases Leads to Dispersal of Pseudomonas aeruginosa Biofilms.Antimicrob Agents Chemother. 2021 Mar 18;65(4):e02431-20. doi: 10.1128/AAC.02431-20. Print 2021 Mar 18. Antimicrob Agents Chemother. 2021. PMID: 33495218 Free PMC article.
-
Differential impact on motility and biofilm dispersal of closely related phosphodiesterases in Pseudomonas aeruginosa.Sci Rep. 2020 Apr 10;10(1):6232. doi: 10.1038/s41598-020-63008-5. Sci Rep. 2020. PMID: 32277108 Free PMC article.
-
Diguanylate Cyclases and Phosphodiesterases Required for Basal-Level c-di-GMP in Pseudomonas aeruginosa as Revealed by Systematic Phylogenetic and Transcriptomic Analyses.Appl Environ Microbiol. 2019 Oct 16;85(21):e01194-19. doi: 10.1128/AEM.01194-19. Print 2019 Nov 1. Appl Environ Microbiol. 2019. PMID: 31444209 Free PMC article.
-
Molecular and structural facets of c-di-GMP signalling associated with biofilm formation in Pseudomonas aeruginosa.Mol Aspects Med. 2021 Oct;81:101001. doi: 10.1016/j.mam.2021.101001. Epub 2021 Jul 24. Mol Aspects Med. 2021. PMID: 34311995 Review.
Cited by
-
NO-induced biofilm dispersion in Pseudomonas aeruginosa is mediated by an MHYT domain-coupled phosphodiesterase.J Bacteriol. 2013 Aug;195(16):3531-42. doi: 10.1128/JB.01156-12. Epub 2013 May 31. J Bacteriol. 2013. PMID: 23729646 Free PMC article.
-
The nitric oxide paradox: antimicrobial and inhibitor of antibiotic efficacy.Emerg Top Life Sci. 2024 Feb 22;8(1):37-43. doi: 10.1042/ETLS20230114. Emerg Top Life Sci. 2024. PMID: 37975610 Free PMC article.
-
Clearance of Pseudomonas aeruginosa foreign-body biofilm infections through reduction of the cyclic Di-GMP level in the bacteria.Infect Immun. 2013 Aug;81(8):2705-13. doi: 10.1128/IAI.00332-13. Epub 2013 May 20. Infect Immun. 2013. PMID: 23690403 Free PMC article.
-
NO donors and NO delivery methods for controlling biofilms in chronic lung infections.Appl Microbiol Biotechnol. 2021 May;105(10):3931-3954. doi: 10.1007/s00253-021-11274-2. Epub 2021 May 3. Appl Microbiol Biotechnol. 2021. PMID: 33937932 Free PMC article. Review.
-
Origin and Impact of Nitric Oxide in Pseudomonas aeruginosa Biofilms.J Bacteriol. 2016 Jan 1;198(1):55-65. doi: 10.1128/JB.00371-15. J Bacteriol. 2016. PMID: 26260455 Free PMC article. Review.
References
-
- Arai, H., T. Kodama, and Y. Igarashi. 1999. Effect of nitrogen oxides on expression of the nir and nor genes for denitrification in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 170:19-24. - PubMed
-
- Aravind, L., V. Anantharaman, and L. M. Iyer. 2003. Evolutionary connections between bacterial and eukaryotic signaling systems: a genomic perspective. Curr. Opin. Microbiol. 6:490-497. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

