Ca2+-independent positive molecular inotropy for failing rabbit and human cardiac muscle by alpha-myosin motor gene transfer
- PMID: 19801488
- PMCID: PMC4048941
- DOI: 10.1096/fj.09-140566
Ca2+-independent positive molecular inotropy for failing rabbit and human cardiac muscle by alpha-myosin motor gene transfer
Abstract
Current inotropic therapies used to increase cardiac contractility of the failing heart center on increasing the amount of calcium available for contraction, but their long-term use is associated with increased mortality due to fatal arrhythmias. Thus, there is a need to develop and explore novel inotropic therapies that can act via calcium-independent mechanisms. The purpose of this study was to determine whether fast alpha-myosin molecular motor gene transfer can confer calcium-independent positive inotropy in slow beta-myosin-dominant rabbit and human failing ventricular myocytes. To this end, we generated a recombinant adenovirus (AdMYH6) to deliver the full-length human alpha-myosin gene to adult rabbit and human cardiac myocytes in vitro. Fast alpha-myosin motor expression was determined by Western blotting and immunocytochemical analysis and confocal imaging. In experiments using electrically stimulated myocytes from ischemic failing hearts, AdMYH6 increased the contractile amplitude of failing human [23.9+/-7.8 nm (n=10) vs. AdMYH6 amplitude 78.4+/-16.5 nm (n=6)] and rabbit myocytes. The intracellular calcium transient amplitude was not altered. Control experiments included the use of a green fluorescent protein or a beta-myosin heavy chain adenovirus. Our data provide evidence for a novel form of calcium-independent positive inotropy in failing cardiac myocytes by fast alpha-myosin motor protein gene transfer.
Figures


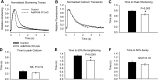
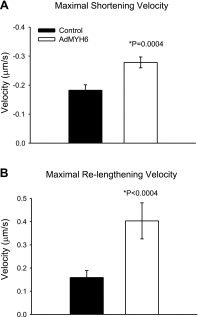



References
-
- Perrone S. V., Kaplinsky E. J. Calcium sensitizer agents: a new class of inotropic agents in the treatment of decompensated heart failure. Int J Cardiol. 2005;103:248–255. - PubMed
-
- Cohn J. N., Goldstein S. O., Greenberg B. H., Lorell B. H., Bourge R. C., Jaski B. E., Gottlieb S. O., McGrew F., 3rd, DeMets D. L., White B. G. A dose-dependent increase in mortality with vesnarinone among patients with severe heart failure: Vesnarinone Trial Investigators. N Engl J Med. 1998;339:1810–1816. - PubMed
-
- Antoniades C., Tousoulis D., Koumallos N., Marinou K., Stefanadis C. Levosimendan: beyond its simple inotropic effect in heart failure. Pharmacol Ther. 2007;114:184–197. - PubMed
-
- Cleland J. G. F., Freemantle N., Coletta A. P., Clark A. L. Clinical trials update from the American Heart Association: REPAIR-AMI, ASTAMI, JELIS, MEGA, REVIVE-II, SURVIVE, and PROACTIVE. Eur J Heart Fail. 2006;8:105–110. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

