Dominant role of antigen dose in CD4+Foxp3+ regulatory T cell induction and expansion
- PMID: 19801514
- PMCID: PMC3142864
- DOI: 10.4049/jimmunol.0901459
Dominant role of antigen dose in CD4+Foxp3+ regulatory T cell induction and expansion
Abstract
The definitions of tolerogenic vs immunogenic dendritic cells (DC) remain controversial. Immature DC have been shown to induce T regulatory cells (Treg) specific for foreign and allogeneic Ags. However, we have previously reported that mature DC (mDC) prevented the onset of autoimmune diabetes, whereas immature DC (iDC) were therapeutically ineffective. In this study, islet-specific CD4(+) T cells from BDC2.5 TCR-transgenic mice were stimulated in the absence of exogenous cytokine with iDC or mDC pulsed with high- or low-affinity antigenic peptides and examined for Treg induction. Both iDC and mDC presenting low peptide doses induced weak TCR signaling via the Akt/mammalian target of rapamycin (mTOR) pathway, resulting in significant expansion of Foxp3(+) Treg. Furthermore, unpulsed mDC, but not iDC, also induced Treg. High peptide doses induced strong Akt/mTOR signaling and favored the expansion of Foxp3(neg) Th cells. The inverse correlation of Foxp3 and Akt/mTOR signaling was also observed in DO11.10 and OT-II TCR-transgenic T cells and was recapitulated with anti-CD3/CD28 stimulation in the absence of DC. IL-6 production in these cultures correlated positively with Ag dose and inversely with Treg expansion. Studies with T cells or DC from IL-6(-/-) mice revealed that IL-6 production by T cells was more important in the inhibition of Treg induction at low Ag doses. These studies indicate that the strength of Akt/mTOR signaling, a critical T cell-intrinsic determinant for Treg vs Th induction, can be controlled by adjusting the dose of antigenic peptide. Furthermore, this operates in a dominant fashion over DC phenotype and cytokine production.
Figures


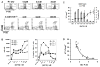


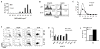
References
-
- Castano L, Eisenbarth GS. Type-I diabetes: a chronic autoimmune disease of human, mouse and rat. Annu Rev Immunol. 1990;8:647–679. - PubMed
-
- Tritt M, Sgouroudis E, d’Hennezel E, Albanese A, Piccirillo CA. Functional waning of naturally occurring CD4+ regulatory T-cells contributes to the onset of autoimmune diabetes. Diabetes. 2008;57:113–123. - PubMed
-
- Gregori S, Giarratana N, Smiroldo S, Adorini L. Dynamics of pathogenic and suppressor T cells in autoimmune diabetes development. J Immunol. 2003;171:4040–4047. - PubMed
-
- Chai JG, Coe D, Chen D, Simpson E, Dyson J, Scott D. In Vitro Expansion Improves In Vivo Regulation by CD4+CD25+ Regulatory T Cells. J Immunol. 2008;180:858–869. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

