The E3 ubiquitin ligase Cbl-b regulates expansion but not functional activity of self-reactive CD4 T cells
- PMID: 19801520
- PMCID: PMC2857710
- DOI: 10.4049/jimmunol.0901243
The E3 ubiquitin ligase Cbl-b regulates expansion but not functional activity of self-reactive CD4 T cells
Abstract
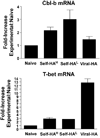
Cbl-b is an E3 ubiquitin ligase that limits Ag responsiveness in T cells by targeting TCR-inducible signaling molecules. Cbl-b deficiency thus renders T cells hyperresponsive to antigenic stimulation and predisposes individuals toward developing autoimmunity. In part because Cbl-b(-/-) T cells do not require CD28 costimulation to become activated, and insufficient costimulation is a critical parameter that confers anergy induction over effector differentiation, it has been hypothesized that Cbl-b(-/-) T cells are resistant to anergy. This possibility has been supported in models in which anergy is normally induced in vitro, or in vivo following exposure to soluble Ag boluses. In the current study, we characterized the response of Cbl-b(-/-) CD4 T cells in an in vivo system in which anergy is normally induced by a constitutively expressed peripheral self-Ag. Cbl-b expression increased in self-Ag-induced anergic wild-type CD4 T cells, and Cbl-b(-/-) CD4 T cells underwent more robust proliferation and expansion upon initially encountering cognate self-Ag compared with wild-type counterparts. Nevertheless, both wild-type and Cbl-b(-/-) CD4 T cells ultimately developed the same impaired ability to respond to antigenic restimulation. The more extensive expansion that occurred during the initial induction of anergy did, however, allow the anergic CD4 T cells to expand to greater numbers when they were functionally resuscitated following replacement of the initial source of tolerizing self-Ag with a viral form of the same Ag.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures






References
-
- Kappler J, Roehm M, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. - PubMed
-
- Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonma-ture CD4+8+ thymocytes. Nature. 1988;333:742–746. - PubMed
-
- Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100–103. - PubMed
-
- Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–1039. - PubMed
-
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

