Adult ceramide synthase 2 (CERS2)-deficient mice exhibit myelin sheath defects, cerebellar degeneration, and hepatocarcinomas
- PMID: 19801672
- PMCID: PMC2785198
- DOI: 10.1074/jbc.M109.031971
Adult ceramide synthase 2 (CERS2)-deficient mice exhibit myelin sheath defects, cerebellar degeneration, and hepatocarcinomas
Abstract
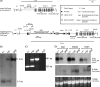
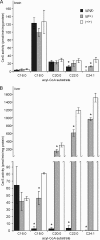
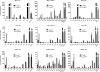
(Dihydro)ceramide synthase 2 (cers2, formerly called lass2) is the most abundantly expressed member of the ceramide synthase gene family, which includes six isoforms in mice. CERS2 activity has been reported to be specific toward very long fatty acid residues (C22-C24). In order to study the biological role of CERS2, we have inactivated its coding region in transgenic mice using gene-trapped embryonic stem cells that express lacZ reporter DNA under control of the cers2 promoter. The resulting mice lack ceramide synthase activity toward C24:1 in the brain as well as the liver and show only very low activity toward C18:0-C22:0 in liver and reduced activity toward C22:0 residues in the brain. In addition, these mice exhibit strongly reduced levels of ceramide species with very long fatty acid residues (>or=C22) in the liver, kidney, and brain. From early adulthood on, myelin stainability is progressively lost, biochemically accompanied by about 50% loss of compacted myelin and 80% loss of myelin basic protein. Starting around 9 months, both the medullary tree and the internal granular layer of the cerebellum show significant signs of degeneration associated with the formation of microcysts. Predominantly in the peripheral nervous system, we observed vesiculation and multifocal detachment of the inner myelin lamellae in about 20% of the axons. Beyond 7 months, the CERS2-deficient mice developed hepatocarcinomas with local destruction of tissue architecture and discrete gaps in renal parenchyma. Our results indicate that CERS2 activity supports different biological functions: maintenance of myelin, stabilization of the cerebellar as well as renal histological architecture, and protection against hepatocarcinomas.
Figures





References
-
- Kolesnick R. N., Goñi F. M., Alonso A. (2000) J. Cell. Physiol. 184, 285–300 - PubMed
-
- Kolter T., Sandhoff K. (1999) Angew. Chem. Int. Ed. 38, 1532–1568 - PubMed
-
- Braun P. E., Snell E. E. (1968) J. Biol. Chem. 243, 3775–3783 - PubMed
-
- Mandon E. C., Ehses I., Rother J., van Echten G., Sandhoff K. (1992) J. Biol. Chem. 267, 11144–11148 - PubMed
-
- Stoffel W., LeKim D., Sticht G. (1968) Hoppe-Seylers Z. Physiol. Chem. 349, 1637–1644 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

