Identification of a new exosite involved in catalytic turnover by the streptokinase-plasmin activator complex during human plasminogen activation
- PMID: 19801674
- PMCID: PMC2781679
- DOI: 10.1074/jbc.M109.046573
Identification of a new exosite involved in catalytic turnover by the streptokinase-plasmin activator complex during human plasminogen activation
Abstract
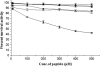
With the goal of identifying hitherto unknown surface exosites of streptokinase involved in substrate human plasminogen recognition and catalytic turnover, synthetic peptides encompassing the 170 loop (CQFTPLNPDDDFRPGLKDTKLLC) in the beta-domain were tested for selective inhibition of substrate human plasminogen activation by the streptokinase-plasmin activator complex. Although a disulfide-constrained peptide exhibited strong inhibition, a linear peptide with the same sequence, or a disulfide-constrained variant with a single lysine to alanine mutation showed significantly reduced capabilities of inhibition. Alanine-scanning mutagenesis of the 170 loop of the beta-domain of streptokinase was then performed to elucidate its importance in streptokinase-mediated plasminogen activation. Some of the 170 loop mutants showed a remarkable decline in k(cat) without any alteration in apparent substrate affinity (K(m)) as compared with wild-type streptokinase and identified the importance of Lys(180) as well as Pro(177) in the functioning of this loop. Remarkably, these mutants were able to generate amidolytic activity and non-proteolytic activation in "partner" plasminogen as wild-type streptokinase. Moreover, cofactor activities of the 170 loop mutants, pre-complexed with plasmin, against microplasminogen as the substrate showed a similar pattern of decline in k(cat) as that observed in the case of full-length plasminogen, with no concomitant change in K(m). These results strongly suggest that the 170 loop of the beta-domain of streptokinase is important for catalysis by the streptokinase-plasmin(ogen) activator complex, particularly in catalytic processing/turnover of substrate, although it does not seem to contribute significantly toward enzyme-substrate affinity per se.
Figures




References
-
- Lijnen H. R., Stassen J. M., Vanlinthout I., Fukao H., Okada K., Matsuo O., Collen D. (1991) Thromb. Haemost. 66, 468–473 - PubMed
-
- McClintock D. K., Bell P. H. (1971) Biochem. Biophys. Res. Commun. 43, 694–702 - PubMed
-
- Bajaj A. P., Castellino F. J. (1977) J. Biol. Chem. 252, 492–498 - PubMed
-
- Boxrud P. D., Verhamme I. M., Bock P. E. (2004) J. Biol. Chem. 279, 36633–36641 - PubMed
-
- Boxrud P. D., Bock P. E. (2004) J. Biol. Chem. 279, 36642–36649 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

