Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands
- PMID: 19801985
- PMCID: PMC2821672
- DOI: 10.1038/ni.1792
Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands
Erratum in
- Nat Immunol. 2010 Jun;11(6):543
Abstract
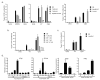
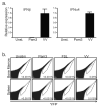
Despite the paradigm that the innate immune system uses nucleic acid-specific receptors to detect viruses because of a lack of other conserved features, many viruses are recognized by Toll-like receptor 2 (TLR2) and TLR4. The relevance of this recognition for antiviral immunity remains largely unexplained. Here we report that TLR2 activation by viruses led to the production of type I interferon. TLR2-dependent induction of type I interferon occurred only in response to viral ligands, which indicates that TLR2 is able to discriminate between pathogen classes. We demonstrate that this specialized response was mediated by Ly6C(hi) inflammatory monocytes. Thus, the innate immune system can detect certain non-nucleic acid features of viruses and links this recognition to the induction of specific antiviral genes.
Figures

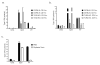



Comment in
-
TLR2 joins the interferon gang.Nat Immunol. 2009 Nov;10(11):1139-41. doi: 10.1038/ni1109-1139. Nat Immunol. 2009. PMID: 19841644
References
-
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. - PubMed
-
- Kawai T, Akira S. Innate immune recognition of viral infection. Nature immunology. 2006;7:131–137. - PubMed
-
- Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25:373–381. - PubMed
-
- van den Broek MF, Muller U, Huang S, Zinkernagel RM, Aguet M. Immune defence in mice lacking type I and/or type II interferon receptors. Immunological reviews. 1995;148:5–18. - PubMed
-
- Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nature reviews. 2008;8:594–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

