Basal forebrain activation enhances cortical coding of natural scenes
- PMID: 19801988
- PMCID: PMC3576925
- DOI: 10.1038/nn.2402
Basal forebrain activation enhances cortical coding of natural scenes
Abstract
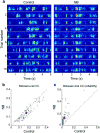
The nucleus basalis of the basal forebrain is an essential component of the neuromodulatory system controlling the behavioral state of an animal and it is thought to be important in regulating arousal and attention. However, the effect of nucleus basalis activation on sensory processing remains poorly understood. Using polytrode recording in rat visual cortex, we found that nucleus basalis stimulation caused prominent decorrelation between neurons and marked improvement in the reliability of neuronal responses to natural scenes. The decorrelation depended on local activation of cortical muscarinic acetylcholine receptors, whereas the increased reliability involved distributed neural circuits, as evidenced by nucleus basalis-induced changes in thalamic responses. Further analysis showed that the decorrelation and increased reliability improved cortical representation of natural stimuli in a complementary manner. Thus, the basal forebrain neuromodulatory circuit, which is known to be activated during aroused and attentive states, acts through both local and distributed mechanisms to improve sensory coding.
Figures



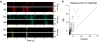


References
-
- Robbins TW. Arousal systems and attentional processes. Biol Psychol. 1997;45:57–71. - PubMed
-
- Jones BE. Modulation of cortical activation and behavioral arousal by cholinergic and orexinergic systems. Ann N Y Acad Sci. 2008;1129:26–34. - PubMed
-
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. - PubMed
-
- Steriade M, McCarley RW. Brainstem control of wakefulness and sleep. Plenum Press; New York: 1990.
-
- Lehmann J, Nagy JI, Atmadia S, Fibiger HC. The nucleus basalis magnocellularis: the origin of a cholinergic projection to the neocortex of the rat. Neuroscience. 1980;5:1161–74. - PubMed

