The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A signaling in metastatic melanoma
- PMID: 19802008
- PMCID: PMC2803338
- DOI: 10.1038/onc.2009.305
The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A signaling in metastatic melanoma
Abstract
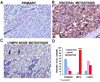
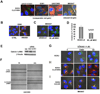
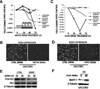
Tyrosine kinase receptors represent targets of great interest for cancer therapy. Here we show, for the first time, the importance of the orphan tyrosine kinase receptor, ROR2, in melanoma progression. Using melanoma tissue microarrays, we show that ROR2 is expressed predominantly in metastatic melanoma. As ROR2 has been shown to specifically interact with the non-canonical Wnt ligand, Wnt5A, this corroborates our earlier data implicating Wnt5A as a mediator of melanoma metastasis. We show here that increases in Wnt5A cause increases in ROR2 expression, as well as the PKC-dependent, clathrin-mediated internalization of ROR2. WNT5A knockdown by siRNA decreases ROR2 expression, but silencing of ROR2 has no effect on WNT5A levels. ROR2 knockdown does, however, result in a decrease in signaling downstream of Wnt5A. Using in vitro and in vivo metastasis assays, we show that ROR2 is necessary for the Wnt5A-mediated metastasis of melanoma cells. These data imply that ROR2 may represent a novel target for melanoma therapy.
Figures



Similar articles
-
Myeloma cells inhibit non-canonical wnt co-receptor ror2 expression in human bone marrow osteoprogenitor cells: effect of wnt5a/ror2 pathway activation on the osteogenic differentiation impairment induced by myeloma cells.Leukemia. 2013 Feb;27(2):451-63. doi: 10.1038/leu.2012.190. Epub 2012 Jul 11. Leukemia. 2013. PMID: 22781592
-
Over-expression of ROR2 and Wnt5a cooperatively correlates with unfavorable prognosis in patients with non-small cell lung cancer.Oncotarget. 2015 Sep 22;6(28):24912-21. doi: 10.18632/oncotarget.4701. Oncotarget. 2015. PMID: 26305508 Free PMC article.
-
Ror2-Src signaling in metastasis of mouse melanoma cells is inhibited by NRAGE.Cancer Genet. 2012 Nov;205(11):552-62. doi: 10.1016/j.cancergen.2012.09.002. Epub 2012 Nov 9. Cancer Genet. 2012. PMID: 23142633
-
Hear the Wnt Ror: how melanoma cells adjust to changes in Wnt.Pigment Cell Melanoma Res. 2009 Dec;22(6):724-39. doi: 10.1111/j.1755-148X.2009.00627.x. Epub 2009 Aug 25. Pigment Cell Melanoma Res. 2009. PMID: 19708915 Free PMC article. Review.
-
Insight into the role of Wnt5a-induced signaling in normal and cancer cells.Int Rev Cell Mol Biol. 2015;314:117-48. doi: 10.1016/bs.ircmb.2014.10.003. Epub 2014 Nov 18. Int Rev Cell Mol Biol. 2015. PMID: 25619716 Review.
Cited by
-
Expression of Ror2 mediates invasive phenotypes in renal cell carcinoma.PLoS One. 2014 Dec 26;9(12):e116101. doi: 10.1371/journal.pone.0116101. eCollection 2014. PLoS One. 2014. PMID: 25542006 Free PMC article.
-
Migration and invasion is inhibited by silencing ROR1 and ROR2 in chemoresistant ovarian cancer.Oncogenesis. 2016 May 30;5(5):e226. doi: 10.1038/oncsis.2016.32. Oncogenesis. 2016. PMID: 27239958 Free PMC article.
-
Protein Depalmitoylation Is Induced by Wnt5a and Promotes Polarized Cell Behavior.J Biol Chem. 2015 Jun 19;290(25):15707-15716. doi: 10.1074/jbc.M115.639609. Epub 2015 May 5. J Biol Chem. 2015. PMID: 25944911 Free PMC article.
-
Demonstration of a WNT5A-IL-6 positive feedback loop in melanoma cells: Dual interference of this loop more effectively impairs melanoma cell invasion.Oncotarget. 2016 Jun 21;7(25):37790-37802. doi: 10.18632/oncotarget.9332. Oncotarget. 2016. PMID: 27191257 Free PMC article.
-
WNT5A induces castration-resistant prostate cancer via CCL2 and tumour-infiltrating macrophages.Br J Cancer. 2018 Mar 6;118(5):670-678. doi: 10.1038/bjc.2017.451. Epub 2018 Jan 30. Br J Cancer. 2018. PMID: 29381686 Free PMC article.
References
-
- Afzal AR, Rajab A, Fenske CD, Oldridge M, Elanko N, Ternes-Pereira E, et al. Recessive Robinow syndrome, allelic to dominant brachydactyly type B, is caused by mutation of ROR2. Nat Genet. 2000;25:419–422. - PubMed
-
- Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406:536–540. - PubMed
-
- Da Forno PD, Pringle JH, Hutchinson P, Osborn J, Huang Q, Potter L, et al. WNT5A expression increases during melanoma progression and correlates with outcome. Clin Cancer Res. 2008;14:5825–5832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

