Field evaluation of two rapid diagnostic tests for Neisseria meningitidis serogroup A during the 2006 outbreak in Niger
- PMID: 19802392
- PMCID: PMC2752163
- DOI: 10.1371/journal.pone.0007326
Field evaluation of two rapid diagnostic tests for Neisseria meningitidis serogroup A during the 2006 outbreak in Niger
Abstract
The Pastorex((R)) (BioRad) rapid agglutination test is one of the main rapid diagnostic tests (RDTs) for meningococcal disease currently in use in the "meningitis belt". Earlier evaluations, performed after heating and centrifugation of cerebrospinal fluid (CSF) samples, under good laboratory conditions, showed high sensitivity and specificity. However, during an epidemic, the test may be used without prior sample preparation. Recently a new, easy-to-use dipstick RDT for meningococcal disease detection on CSF was developed by the Centre de Recherche Médicale et Sanitaire in Niger and the Pasteur Institute in France. We estimate diagnostic accuracy in the field during the 2006 outbreak of Neisseria meningitidis serogroup A in Maradi, Niger, for the dipstick RDT and Pastorex((R)) on unprepared CSF, (a) by comparing each test's sensitivity and specificity with previously reported values; and (b) by comparing results for each test on paired samples, using McNemar's test. We also (c) estimate diagnostic accuracy of the dipstick RDT on diluted whole blood. We tested unprepared CSF and diluted whole blood from 126 patients with suspected meningococcal disease presenting at four health posts. (a) Pastorex((R)) sensitivity (69%; 95%CI 57-79) was significantly lower than found previously for prepared CSF samples [87% (81-91); or 88% (85-91)], as was specificity [81% (95%CI 68-91) vs 93% (90-95); or 93% (87-96)]. Sensitivity of the dipstick RDT [89% (95%CI 80-95)] was similar to previously reported values for ideal laboratory conditions [89% (84-93) and 94% (90-96)]. Specificity, at 62% (95%CI 48-75), was significantly lower than found previously [94% (92-96) and 97% (94-99)]. (b) McNemar's test for the dipstick RDT vs Pastorex((R)) was statistically significant (p<0.001). (c) The dipstick RDT did not perform satisfactorily on diluted whole blood (sensitivity 73%; specificity 57%).Sensitivity and specificity of Pastorex((R)) without prior CSF preparation were poorer than previously reported results from prepared samples; therefore we caution against using this test during an epidemic if sample preparation is not possible. For the dipstick RDT, sensitivity was similar to, while specificity was not as high as previously reported during a more stable context. Further studies are needed to evaluate its field performance, especially for different populations and other serogroups.
Conflict of interest statement
Figures

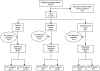
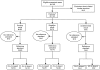
References
-
- Chanteau S, Rose AM, Djibo S, Nato F, Boisier P. Biological diagnosis of meningococcal meningitis in the African meningitis belt: Current epidemic strategy and new perspectives. Vaccine. 2007;25(Supplement 1):A30–A36. - PubMed
-
- Borel T, Rose AM, Guillerm M, Sidikou F, Gerstl S, et al. High sensitivity and specificity of the Pastorex latex agglutination test for Neisseria meningitidis serogroup A during a clinical trial in Niger. Trans R Soc Trop Med Hyg. 2006;100(10):964–969. - PubMed
-
- Djibo S, Njanpop Lafourcade BM, Boisier P, Moussa A, Kobo G, et al. Evaluation of the Pastorex meningitis kit for the rapid identification of Neisseria meningitidis serogroups A and W135. Trans R Soc Trop Med Hyg. 2006;100(6):573–578. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

