The Youth-Nominated Support Team-Version II for suicidal adolescents: A randomized controlled intervention trial
- PMID: 19803568
- PMCID: PMC3319347
- DOI: 10.1037/a0016552
The Youth-Nominated Support Team-Version II for suicidal adolescents: A randomized controlled intervention trial
Abstract
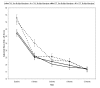
The purpose of this study was to examine the efficacy of the Youth-Nominated Support Team-Version II (YST-II) for suicidal adolescents, an intervention based on social support and health behavior models, which was designed to supplement standard treatments. Psychiatrically hospitalized and suicidal adolescents, 13-17 years of age, were randomly assigned to treatment-as-usual (TAU) + YST-II (n = 223) or TAU only (n = 225). YST-II provided tailored psychoeducation to youth-nominated adults in addition to weekly check-ins for 3 months following hospitalization. In turn, these adults had regular supportive contact with adolescents. Adolescents assigned to TAU + YST-II had an average of 3.43 (SD = 0.83) nominated adults. Measures included the Suicidal Ideation Questionnaire-Junior (SIQ-JR; W. M. Reynolds, 1988), Children's Depression Rating Scale-Revised (E. O. Poznanski & H. B. Mokros, 1996), Beck Hopelessness Scale (A. T. Beck & R. A. Steer, 1993), and Child and Adolescent Functional Assessment Scale (CAFAS; K. Hodges, 1996). YST-II had very limited positive effects, which were moderated by history of multiple suicide attempts, and no negative effects. It resulted in more rapid decreases in suicidal ideation (SIQ-JR) for multiple suicide attempters during the initial 6 weeks after hospitalization (small-to-moderate effect size). For nonmultiple attempters, it was associated with greater declines in functional impairment (CAFAS) at 3 and 12 months (small effect sizes). YST-II had no effects on suicide attempts and no enduring effects on SIQ-JR scores.
(c) 2009 APA, all rights reserved.
Figures
References
-
- Achenbach TM. Manual for the Youth Self-Report and 1991 Profile. University of Vermont, Department of Psychiatry; Burlington, VT: 1991.
-
- Asarnow JR, Jaycox LH, Duan N, LaBorde AP, Rea MM, Murray P, et al. Effectiveness of a Quality Improvement Intervention for Adolescent Depression in Primary Care Clinics: A Randomized Controlled Trial. JAMA: Journal of the American Medical Association. 2005;293(3):311–319. - PubMed
-
- Beck AT, Steer RA. Beck Hopelessness Scale Manual. Harcourt Brace; San Antonio, TX: 1993.
-
- Brent DA, Kolko DJ, Wartella ME, Boylan MB. Adolescent psychiatric inpatients' risk of suicide attempt at 6-month follow-up. Journal of the American Academy of Child & Adolescent Psychiatry. 1993;32(1):95–105. - PubMed
-
- Brown RM, Dahlen E, Mills C, Rick J, Biblarz A. Evaluation of an evolutionary model of self-preservation and self-destruction. Suicide & Life-Threatening Behavior. 1999;29(1):58–71. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials