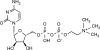The citicoline brain injury treatment (COBRIT) trial: design and methods
- PMID: 19803786
- PMCID: PMC2824223
- DOI: 10.1089/neu.2009.1015
The citicoline brain injury treatment (COBRIT) trial: design and methods
Abstract
Traumatic brain injury (TBI) is a major cause of death and disability. In the United States alone approximately 1.4 million sustain a TBI each year, of which 50,000 people die, and over 200,000 are hospitalized. Despite numerous prior clinical trials no standard pharmacotherapy for the treatment of TBI has been established. Citicoline, a naturally occurring endogenous compound, offers the potential of neuroprotection, neurorecovery, and neurofacilitation to enhance recovery after TBI. Citicoline has a favorable side-effect profile in humans and several meta-analyses suggest a benefit of citicoline treatment in stroke and dementia. COBRIT is a randomized, double-blind, placebo-controlled, multi-center trial of the effects of 90 days of citicoline on functional outcome in patients with complicated mild, moderate, and severe TBI. In all, 1292 patients will be recruited over an estimated 32 months from eight clinical sites with random assignment to citicoline (1000 mg twice a day) or placebo (twice a day), administered enterally or orally. Functional outcomes are assessed at 30, 90, and 180 days after the day of randomization. The primary outcome consists of a set of measures that will be analyzed as a composite measure using a global test procedure at 90 days. The measures comprise the following core battery: the California Verbal Learning Test II; the Controlled Oral Word Association Test; Digit Span; Extended Glasgow Outcome Scale; the Processing Speed Index; Stroop Test part 1 and Stroop Test part 2; and Trail Making Test parts A and B. Secondary outcomes include survival, toxicity, and rate of recovery.
Figures
References
-
- Adibhatla R.M. Hatcher J.F. Citicoline mechanisms and clinical efficacy in cerebral ischemia. J. Neurosci. Res. 2002;70:133–139. - PubMed
-
- Adibhatla R.M. Hatcher J.F. Dempsey R.J. Effects of citicoline on phospholipid and glutathione levels in transient cerebral ischemia. Stroke. 2001;32:2376–2381. - PubMed
-
- Adibhatla R. Hatcher J. Larsen E. Chen X. Sun D. Tsao F. CDP-choline significantly restores phophatuidylcholine levels by differentially affecting phospholipase A2 and CTP: phosphocholinecytidylytransferase after stroke. J Biol Chem. 2006;28:6718–25. - PubMed
-
- Alexandrov A. Citicoline Curr Opinion Invest Drugs. 2001;2:1757–1762. - PubMed
-
- Alkan T. Kahveci N. Goren B. Korfali E. Ozluk K. Ischemic brain injury caused by interrupted versus uninterrupted occlusion in hypotensive rats with subarachnoid hemorrhage: neuroprotective effects of citicoline. Arch. Physiol. Biochem. 2001;109:161–167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical