Chemokine receptor expression and functional effects of chemokines on B cells: implication in the pathogenesis of rheumatoid arthritis
- PMID: 19804625
- PMCID: PMC2787286
- DOI: 10.1186/ar2823
Chemokine receptor expression and functional effects of chemokines on B cells: implication in the pathogenesis of rheumatoid arthritis
Abstract
Introduction: Accumulation of B cells in the rheumatoid arthritis (RA) synovium has been reported, and it has been thought that these cells might contribute to the pathogenesis of RA by antigen presentation, autoantibody production, and/or inflammatory cytokine production. Chemokines could enhance the accumulation of B cells in the synovium. The aims of this study were to determine chemokine receptor expression by B cells both in the peripheral blood of normal donors and subjects with RA, and at the inflammatory site in RA, and the effects of chemokines on B cell activation.
Methods: Cell surface molecule expression was analyzed by flow cytometry. Cellular migration was assessed using chemotaxis chambers. Cellular proliferation was examined by 3H-thymidine incorporation. Tumor necrosis factor (TNF) production was assayed by enzyme-linked immunosorbent assay.
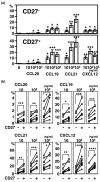
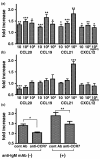
Results: Significant numbers of peripheral blood B cells of healthy donors and subjects with RA expressed CC chemokine receptor (CCR)5 and CXCR3, and most B cells expressed CCR6, CCR7, CXCR4 and CXCR5. CCR5 expression was more frequent on CD27+ than CD27- peripheral blood B cells of healthy donors and RA. Synovial B cells more frequently expressed CCR5, but less often expressed CCR6, CCR7 and CXCR5 compared to peripheral blood in RA. Further functional analyses were performed on peripheral blood B cells from healthy donors. Migration of peripheral blood B cells, especially CD27+ B cells, was enhanced by CC chemokine ligand (CCL)20, CCL19, CCL21 and CXCL12. All four chemokines alone induced B cell proliferation; with CCL21 being the most effective. CCL21 also enhanced the proliferation of anti-immunoglobulin (Ig)M-stimulated B cells and blockade of CCR7 inhibited this effect. CCL20, CCL21 and CXCL12 enhanced TNF production by anti-IgM mAb-stimulated B cells. Finally, stimulation with CXCL12, but not CCL20, CCL19 and CCL21, enhanced inducible costimulator-ligand (ICOSL) expression by peripheral blood B cells of healthy donors and RA, but did not increase B cell-activating factor receptor or transmembrane activator and CAML-interactor.
Conclusions: The data suggest that CCR5, CCR6, CCR7, CXCR3, CXCR4 and CXCR5 may be important for the B cell migration into the synovium of RA patients, and also their local proliferation, cytokine production and ICOSL expression in the synovium.
Figures


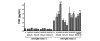

References
-
- Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, Keystone EC, Loveless JE, Burmester GR, Cravets MW, Hessey EW, Shaw T, Totoritis MC. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793–2806. doi: 10.1002/art.22025. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

