Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit
- PMID: 19804761
- PMCID: PMC2812434
- DOI: 10.1016/j.cell.2009.08.027
Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit
Abstract
GABAergic interneurons are key elements in neural coding, but the mechanisms that assemble inhibitory circuits remain unclear. In the spinal cord, the transfer of sensory signals to motor neurons is filtered by GABAergic interneurons that act presynaptically to inhibit sensory transmitter release and postsynaptically to inhibit motor neuron excitability. We show here that the connectivity and synaptic differentiation of GABAergic interneurons that mediate presynaptic inhibition is directed by their sensory targets. In the absence of sensory terminals these GABAergic neurons shun other available targets, fail to undergo presynaptic differentiation, and withdraw axons from the ventral spinal cord. A sensory-specific source of brain derived neurotrophic factor induces synaptic expression of the GABA synthetic enzyme GAD65--a defining biochemical feature of this set of interneurons. The organization of a GABAergic circuit that mediates presynaptic inhibition in the mammalian CNS is therefore controlled by a stringent program of sensory recognition and signaling.
Figures

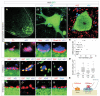
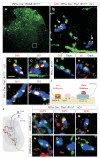
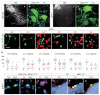

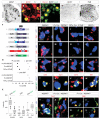
References
-
- Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell. 2000;101:485–498. - PubMed
-
- Burry RW. Development of apparent presynaptic elements formed in response to polylysine coated surfaces. Brain Res. 1982;247:1–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

