Recent advances and new opportunities in lung mechanobiology
- PMID: 19804885
- PMCID: PMC2813412
- DOI: 10.1016/j.jbiomech.2009.09.015
Recent advances and new opportunities in lung mechanobiology
Abstract
Lung function is inextricably linked to mechanics. On short timescales every breath generates dynamic cycles of cell and matrix stretch, along with convection of fluids in the airways and vasculature. Perturbations such airway smooth muscle shortening or surfactant dysfunction rapidly alter respiratory mechanics, with profound influence on lung function. On longer timescales, lung development, maturation, and remodeling all strongly depend on cues from the mechanical environment. Thus mechanics has long played a central role in our developing understanding of lung biology and respiratory physiology. This concise review focuses on progress over the past 5 years in elucidating the molecular origins of lung mechanical behavior, and the cellular signaling events triggered by mechanical perturbations that contribute to lung development, homeostasis, and injury. Special emphasis is placed on the tools and approaches opening new avenues for investigation of lung behavior at integrative cellular and molecular scales. We conclude with a brief summary of selected opportunities and challenges that lie ahead for the lung mechanobiology research community.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures
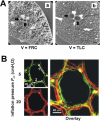
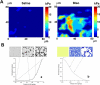

References
-
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–8. - PubMed
-
- Mechanisms and limits of induced postnatal lung growth. Am J Respir Crit Care Med. 2004;170:319–43. - PubMed
-
- Altemeier WA, Matute-Bello G, Frevert CW, Kawata Y, Kajikawa O, Martin TR, Glenny RW. Mechanical ventilation with moderate tidal volumes synergistically increases lung cytokine response to systemic endotoxin. Am J Physiol Lung Cell Mol Physiol. 2004;287:L533–42. - PubMed
-
- Altemeier WA, Matute-Bello G, Gharib SA, Glenny RW, Martin TR, Liles WC. Modulation of lipopolysaccharide-induced gene transcription and promotion of lung injury by mechanical ventilation. J Immunol. 2005;175:3369–76. - PubMed
-
- An SS, Bai TR, Bates JH, Black JL, Brown RH, Brusasco V, Chitano P, Deng L, Dowell M, Eidelman DH, Fabry B, Fairbank NJ, Ford LE, Fredberg JJ, Gerthoffer WT, Gilbert SH, Gosens R, Gunst SJ, Halayko AJ, Ingram RH, Irvin CG, James AL, Janssen LJ, King GG, Knight DA, Lauzon AM, Lakser OJ, Ludwig MS, Lutchen KR, Maksym GN, Martin JG, Mauad T, McParland BE, Mijailovich SM, Mitchell HW, Mitchell RW, Mitzner W, Murphy TM, Pare PD, Pellegrino R, Sanderson MJ, Schellenberg RR, Seow CY, Silveira PS, Smith PG, Solway J, Stephens NL, Sterk PJ, Stewart AG, Tang DD, Tepper RS, Tran T, Wang L. Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. Eur Respir J. 2007;29:834–60. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

