Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: Implications for glaucoma
- PMID: 19805021
- PMCID: PMC2726400
- DOI: 10.1073/pnas.0906678106
Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: Implications for glaucoma
Abstract
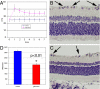
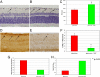
Elevated intraocular pressure (IOP) in glaucoma causes loss of retinal ganglion cells (RGCs) and damage to the optic nerve. Although IOP is controlled pharmacologically, no treatment is available to restore retinal and optic nerve function. We evaluated the effects of NGF eye drops in a rat model of glaucoma. We also treated 3 patients with progressive visual field defects despite IOP control. Glaucoma was induced in rats through injection of hypertonic saline into the episcleral vein. Initially, 2 doses of NGF (100 and 200 mug/mL) were tested on 24 rats, and the higher dose was found to be more effective. Glaucoma was then induced in an additional 36 rats: half untreated and half treated with 200 mug/mL NGF QID for 7 weeks. Apoptosis/survival of RGCs was evaluated by histological, biochemical, and molecular analysis. Three patients with advanced glaucoma underwent psychofunctional and electrofunctional tests at baseline, after 3 months of NGF eye drops, and after 3 months of follow-up. Seven weeks of elevated IOP caused RGC degeneration resulting in 40% cell death. Significantly less RGC loss was observed with NGF treatment (2,530 +/- 121 vs. 1,850 +/- 156 RGCs/mm(2)) associated with inhibition of cell death by apoptosis. Patients treated with NGF demonstrated long lasting improvements in visual field, optic nerve function, contrast sensitivity, and visual acuity. NGF exerted neuroprotective effects, inhibiting apoptosis of RGCs in animals with glaucoma. In 3 patients with advanced glaucoma, treatment with topical NGF improved all parameters of visual function. These results may open therapeutic perspectives for glaucoma and other neurodegenerative diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

