SH3TC2/KIAA1985 protein is required for proper myelination and the integrity of the node of Ranvier in the peripheral nervous system
- PMID: 19805030
- PMCID: PMC2765159
- DOI: 10.1073/pnas.0905523106
SH3TC2/KIAA1985 protein is required for proper myelination and the integrity of the node of Ranvier in the peripheral nervous system
Erratum in
- Proc Natl Acad Sci U S A. 2010 Aug 24;107(34):15305. Kleine, Henning [added]; Luscher, Bernhard [added]
Abstract
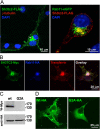
Charcot-Marie-Tooth disease type 4C (CMT4C) is an early-onset, autosomal recessive form of demyelinating neuropathy. The clinical manifestations include progressive scoliosis, delayed age of walking, muscular atrophy, distal weakness, and reduced nerve conduction velocity. The gene mutated in CMT4C disease, SH3TC2/KIAA1985, was recently identified; however, the function of the protein it encodes remains unknown. We have generated knockout mice where the first exon of the Sh3tc2 gene is replaced with an enhanced GFP cassette. The Sh3tc2(DeltaEx1/DeltaEx1) knockout animals develop progressive peripheral neuropathy manifested by decreased motor and sensory nerve conduction velocity and hypomyelination. We show that Sh3tc2 is specifically expressed in Schwann cells and localizes to the plasma membrane and to the perinuclear endocytic recycling compartment, concordant with its possible function in myelination and/or in regions of axoglial interactions. Concomitantly, transcriptional profiling performed on the endoneurial compartment of peripheral nerves isolated from control and Sh3tc2(DeltaEx1/DeltaEx1) animals uncovered changes in transcripts encoding genes involved in myelination and cell adhesion. Finally, detailed analyses of the structures composed of compact and noncompact myelin in the peripheral nerve of Sh3tc2(DeltaEx1/DeltaEx1) animals revealed abnormal organization of the node of Ranvier, a phenotype that we confirmed in CMT4C patient nerve biopsies. The generated Sh3tc2 knockout mice thus present a reliable model of CMT4C neuropathy that was instrumental in establishing a role for Sh3tc2 in myelination and in the integrity of the node of Ranvier, a morphological phenotype that can be used as an additional CMT4C diagnostic marker.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Skre H. Genetic and clinical aspects of Charcot-Marie-Tooth's disease. Clin Genet. 1974;6:98–118. - PubMed
-
- Gabreels-Festen AA, Gabreels FJ, Jennekens FG, Joosten EM, Janssen-van Kempen TW. Autosomal recessive form of hereditary motor and sensory neuropathy type I. Neurology. 1992;42:1755–1761. - PubMed
-
- Kessali M, et al. A clinical, electrophysiologic, neuropathologic, and genetic study of two large Algerian families with an autosomal recessive demyelinating form of Charcot-Marie-Tooth disease. Neurology. 1997;48:867–873. - PubMed
-
- Houlden H, et al. The phenotype of Charcot-Marie-Tooth disease type 4C due to SH3TC2 mutations and possible predisposition to an inflammatory neuropathy. Neuromuscul Disord. 2009;19:264–269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

