Glucocorticoid regulation of the circadian clock modulates glucose homeostasis
- PMID: 19805059
- PMCID: PMC2757402
- DOI: 10.1073/pnas.0909733106
Glucocorticoid regulation of the circadian clock modulates glucose homeostasis
Abstract
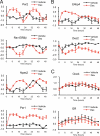
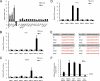
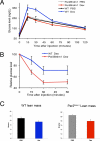
Circadian clock genes are regulated by glucocorticoids; however, whether this regulation is a direct or secondary effect and the physiological consequences of this regulation were unknown. Here, we identified glucocorticoid response elements (GREs) at multiple clock genes and showed that 3 were directly regulated by the glucocorticoid receptor. We determined that a GRE within the core clock gene Per2 was continuously occupied during rhythmic expression and essential for glucocorticoid regulation of that gene in vivo. We further demonstrated that mice with a genomic deletion spanning this GRE expressed elevated leptin levels and were protected from glucose intolerance and insulin resistance on glucocorticoid treatment but not from muscle wasting. We conclude that Per2 is an integral component of a particular glucocorticoid regulatory pathway and that glucocorticoid regulation of the peripheral clock is selectively required for some actions of glucocorticoids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
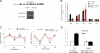


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

