A wound-induced Wnt expression program controls planarian regeneration polarity
- PMID: 19805089
- PMCID: PMC2743725
- DOI: 10.1073/pnas.0906823106
A wound-induced Wnt expression program controls planarian regeneration polarity
Abstract
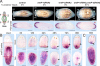
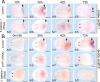
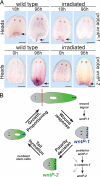
Regeneration requires specification of the identity of new tissues to be made. Whether this process relies only on intrinsic regulative properties of regenerating tissues or whether wound signaling provides input into tissue repatterning is not known. The head-versus-tail regeneration polarity decision in planarians, which requires Wnt signaling, provides a paradigm to study the process of tissue identity specification during regeneration. The Smed-wntP-1 gene is required for regeneration polarity and is expressed at the posterior pole of intact animals. Surprisingly, wntP-1 was expressed at both anterior- and posterior-facing wounds rapidly after wounding. wntP-1 expression was induced by all types of wounds examined, regardless of whether wounding prompted tail regeneration. Regeneration polarity was found to require new expression of wntP-1. Inhibition of the wntP-2 gene enhanced the polarity phenotype due to wntP-1 inhibition, with new expression of wntP-2 in regeneration occurring subsequent to expression of wntP-1 and localized only to posterior-facing wounds. New expression of wntP-2 required wound-induced wntP-1. Finally, wntP-1 and wntP-2 expression changes occurred even in the absence of neoblast stem cells, which are required for regeneration, suggesting that the role of these genes in polarity is independent of and instructive for tail formation. These data indicate that wound-induced input is involved in resetting the normal polarized features of the body axis during regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Brockes JP, Kumar A. Comparative aspects of animal regeneration. Annu Rev Cell Dev Biol. 2008;24:525–549. - PubMed
-
- Harrison RG. Experiments on the development of the fore-limb of Amblystoma, a self-differentiating equipotential system. J Exp Zool. 1918;25:413–461.
-
- Reddien PW, Bermange AL, Kicza AM, Sánchez Alvarado A. BMP signaling regulates the dorsal planarian midline and is needed for asymmetric regeneration. Development. 2007;134:4043–4051. - PubMed
-
- Molina MD, Saló E, Cebrià F. The BMP pathway is essential for re-specification and maintenance of the dorsoventral axis in regenerating and intact planarians. Dev Biol. 2007;311:79–94. - PubMed
-
- Whitehead GG, Makino S, Lien CL, Keating MT. fgf20 is essential for initiating zebrafish fin regeneration. Science. 2005;310:1957–1960. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

