Photolysis of iron-siderophore chelates promotes bacterial-algal mutualism
- PMID: 19805106
- PMCID: PMC2761308
- DOI: 10.1073/pnas.0905512106
Photolysis of iron-siderophore chelates promotes bacterial-algal mutualism
Abstract
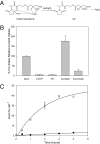
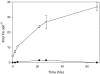
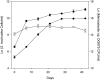
Marine microalgae support world fisheries production and influence climate through various mechanisms. They are also responsible for harmful blooms that adversely impact coastal ecosystems and economies. Optimal growth and survival of many bloom-forming microalgae, including climatically important dinoflagellates and coccolithophores, requires the close association of specific bacterial species, but the reasons for these associations are unknown. Here, we report that several clades of Marinobacter ubiquitously found in close association with dinoflagellates and coccolithophores produce an unusual lower-affinity dicitrate siderophore, vibrioferrin (VF). Fe-VF chelates undergo photolysis at rates that are 10-20 times higher than siderophores produced by free-living marine bacteria, and unlike the latter, the VF photoproduct has no measurable affinity for iron. While both an algal-associated bacterium and a representative dinoflagellate partner, Scrippsiella trochoidea, used iron from Fe-VF chelates in the dark, in situ photolysis of the chelates in the presence of attenuated sunlight increased bacterial iron uptake by 70% and algal uptake by >20-fold. These results suggest that the bacteria promote algal assimilation of iron by facilitating photochemical redox cycling of this critical nutrient. Also, binary culture experiments and genomic evidence suggest that the algal cells release organic molecules that are used by the bacteria for growth. Such mutualistic sharing of iron and fixed carbon has important implications toward our understanding of the close beneficial interactions between marine bacteria and phytoplankton, and the effect of these interactions on algal blooms and climate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Coale KH, et al. A massive phytoplankton bloom induced by an ecosystem-scale iron fertilization experiment in the equatorial Pacific Ocean. Nature. 1996;383:495–501. - PubMed
-
- Dymond J, Lyle M. Flux comparisons between sediments and sediment traps in the eastern tropical Pacific: Implications for atmospheric CO2 variations during the Pleistocene. Limnol Oceanogr. 1985;30:699–712.
-
- Keller MD, Bellows WK, Guillard RRL. Dimethyl sulfide production in marine phytoplankton. In: Saltzman ES, Cooper WJ, editors. Biogenic Sulfur in the Environment. Washington, DC: Am Chem Soc; 1989. pp. 167–182.
-
- Cloern JE. Our evolving conceptual model of the coastal eutrophication problem. Mar Ecol Prog Ser. 2001;210:223–253.
-
- Cho BC, Azam F. Major role of bacteria in biogeochemical fluxes in the ocean's interior. Nature. 1988;332:441–443.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

