DNA polymerase epsilon and delta proofreading suppress discrete mutator and cancer phenotypes in mice
- PMID: 19805137
- PMCID: PMC2761330
- DOI: 10.1073/pnas.0907147106
DNA polymerase epsilon and delta proofreading suppress discrete mutator and cancer phenotypes in mice
Abstract
Organisms require faithful DNA replication to avoid deleterious mutations. In yeast, replicative leading- and lagging-strand DNA polymerases (Pols epsilon and delta, respectively) have intrinsic proofreading exonucleases that cooperate with each other and mismatch repair to limit spontaneous mutation to less than 1 per genome per cell division. The relationship of these pathways in mammals and their functions in vivo are unknown. Here we show that mouse Pol epsilon and delta proofreading suppress discrete mutator and cancer phenotypes. We found that inactivation of Pol epsilon proofreading elevates base-substitution mutations and accelerates a unique spectrum of spontaneous cancers; the types of tumors are entirely different from those triggered by loss of Pol delta proofreading. Intercrosses of Pol epsilon-, Pol delta-, and mismatch repair-mutant mice show that Pol epsilon and delta proofreading act in parallel pathways to prevent spontaneous mutation and cancer. These findings distinguish Pol epsilon and delta functions in vivo and reveal tissue-specific requirements for DNA replication fidelity.
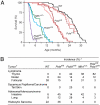
Figures

References
-
- Friedberg EC, et al. DNA Repair and Mutagenesis. 2nd Ed. Washington, D.C.: ASM Press; 2006.
-
- Bernad A, Blanco L, Lázaro JM, Martin G, Salas M. A conserved 3′→5′ exonuclease active site in prokaryotic and eukaryotic DNA polymerases. Cell. 1989;59:219–228. - PubMed
-
- Shevelev IV, Hübscher U. The 3′–5′ exonucleases. Nat Rev Mol Cell Biol. 2002;3:364–376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

