AnkyrinG is required to maintain axo-dendritic polarity in vivo
- PMID: 19805144
- PMCID: PMC2765162
- DOI: 10.1073/pnas.0909267106
AnkyrinG is required to maintain axo-dendritic polarity in vivo
Abstract
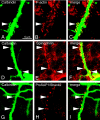
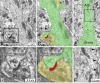
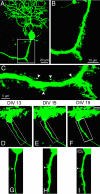
Neurons are highly polarized cells that extend a single axon and several dendrites. Studies with cultured neurons indicate that the proximal portion of the axon, denoted as the axon initial segment (AIS), maintains neuronal polarity in vitro. The membrane-adaptor protein ankyrinG (ankG) is an essential component of the AIS. To determine the relevance of ankG for neuronal polarity in vivo, we studied mice with a cerebellum-specific ankG deficiency. Strikingly, ankG-depleted axons develop protrusions closely resembling dendritic spines. Such axonal spines are enriched with postsynaptic proteins, including ProSAP1/Shank2 and ionotropic and metabotropic glutamate receptors. In addition, immunofluorescence indicated that axonal spines are contacted by presynaptic glutamatergic boutons. For further analysis, double mutants were obtained by crossbreeding ankG(-/-) mice with L7/Purkinje cell-specific promoter 2 (PCP2) mice expressing enhanced green fluorescent protein (EGFP) in Purkinje cells (PCs). This approach allowed precise confocal microscopic mapping of EGFP-positive spiny axons and their subsequent identification at the electron microscopic level. Ultrastructurally, axonal spines contained a typical postsynaptic density and established asymmetric excitatory synapses with presynaptic boutons containing synaptic vesicles. In the shaft of spiny axons, typical ultrastructural features of the AIS, including the membrane-associated dense undercoating and cytoplasmic bundles of microtubules, were absent. Finally, using time-lapse imaging of organotypic cerebellar slice cultures, we demonstrate that nonspiny PC axons of EGFP-positive/ankG(-/-) mice acquire a spiny phenotype within a time range of only 3 days. Collectively, these findings demonstrate that axons of ankG-deficient mice acquire hallmark features of dendrites. AnkG thus is important for maintaining appropriate axo-dendritic polarity in vivo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Craig AM, Banker G. Neuronal polarity. Annu Rev Neurosci. 1994;17:267–310. - PubMed
-
- Winckler B, Mellman I. Neuronal polarity: Controlling the sorting and diffusion of membrane components. Neuron. 1999;23:637–640. - PubMed
-
- Horton AC, Ehlers MD. Neuronal polarity and trafficking. Neuron. 2003;40:277–295. - PubMed
-
- Arimura N, Kaibuchi K. Neuronal polarity: From extracellular signals to intracellular mechanisms. Nat Rev Neurosci. 2007;8:194–205. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

