Evidence for biological nitrification inhibition in Brachiaria pastures
- PMID: 19805171
- PMCID: PMC2752401
- DOI: 10.1073/pnas.0903694106
Evidence for biological nitrification inhibition in Brachiaria pastures
Abstract
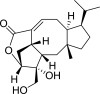
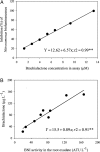
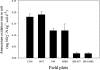
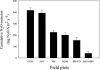
Nitrification, a key process in the global nitrogen cycle that generates nitrate through microbial activity, may enhance losses of fertilizer nitrogen by leaching and denitrification. Certain plants can suppress soil-nitrification by releasing inhibitors from roots, a phenomenon termed biological nitrification inhibition (BNI). Here, we report the discovery of an effective nitrification inhibitor in the root-exudates of the tropical forage grass Brachiaria humidicola (Rendle) Schweick. Named "brachialactone," this inhibitor is a recently discovered cyclic diterpene with a unique 5-8-5-membered ring system and a gamma-lactone ring. It contributed 60-90% of the inhibitory activity released from the roots of this tropical grass. Unlike nitrapyrin (a synthetic nitrification inhibitor), which affects only the ammonia monooxygenase (AMO) pathway, brachialactone appears to block both AMO and hydroxylamine oxidoreductase enzymatic pathways in Nitrosomonas. Release of this inhibitor is a regulated plant function, triggered and sustained by the availability of ammonium (NH(4)(+)) in the root environment. Brachialactone release is restricted to those roots that are directly exposed to NH(4)(+). Within 3 years of establishment, Brachiaria pastures have suppressed soil nitrifier populations (determined as amoA genes; ammonia-oxidizing bacteria and ammonia-oxidizing archaea), along with nitrification and nitrous oxide emissions. These findings provide direct evidence for the existence and active regulation of a nitrification inhibitor (or inhibitors) release from tropical pasture root systems. Exploiting the BNI function could become a powerful strategy toward the development of low-nitrifying agronomic systems, benefiting both agriculture and the environment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Galloway JN, et al. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science. 2008;320:889–892. - PubMed
-
- Dinnes DL, et al. Nitrogen management strategies to reduce nitrate leaching in tile drained Midwestern soils. Agron J. 2002;94:153–171.
-
- Bellamy PH, Loveland PJ, Ian Bradley R, Murray Lark R, Kirk GJD. Carbon losses from all soils across England and Wales 1978–2003. Nature. 2005;437:245–248. - PubMed
-
- Bremner JM, Blackmer AM. Nitrous oxide: Emission from soils during nitrification of fertilizer nitrogen. Science. 1978;199:295–296. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

