CKIepsilon/delta-dependent phosphorylation is a temperature-insensitive, period-determining process in the mammalian circadian clock
- PMID: 19805222
- PMCID: PMC2736905
- DOI: 10.1073/pnas.0908733106
CKIepsilon/delta-dependent phosphorylation is a temperature-insensitive, period-determining process in the mammalian circadian clock
Abstract
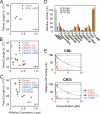
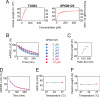
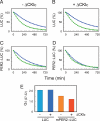
A striking feature of the circadian clock is its flexible yet robust response to various environmental conditions. To analyze the biochemical processes underlying this flexible-yet-robust characteristic, we examined the effects of 1,260 pharmacologically active compounds in mouse and human clock cell lines. Compounds that markedly (>10 s.d.) lengthened the period in both cell lines, also lengthened it in central clock tissues and peripheral clock cells. Most compounds inhibited casein kinase Iepsilon (CKIepsilon) or CKIdelta phosphorylation of the PER2 protein. Manipulation of CKIepsilon/delta-dependent phosphorylation by these compounds lengthened the period of the mammalian clock from circadian (24 h) to circabidian (48 h), revealing its high sensitivity to chemical perturbation. The degradation rate of PER2, which is regulated by CKIepsilon/delta-dependent phosphorylation, was temperature-insensitive in living clock cells, yet sensitive to chemical perturbations. This temperature-insensitivity was preserved in the CKIepsilon/delta-dependent phosphorylation of a synthetic peptide in vitro. Thus, CKIepsilon/delta-dependent phosphorylation is likely a temperature-insensitive period-determining process in the mammalian circadian clock.
Figures

References
-
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. - PubMed
-
- Dunlap JC, Loros JJ, DeCoursey PJ, editors. Chronobiology: Biological Timekeeping. Sunderland, MA: Sinauer Associates; 2004.
-
- Aschoff J. Exogenous and endogenous components in circadian rhythms. Cold Spring Harb Symp Quant Biol. 1960;25:11–28. - PubMed
-
- Aschoff J. Circadian Clocks. In: Aschoff J, editor. North-Holland, Amsterdam: 1965. pp. 95–111.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

