Secretin as a neurohypophysial factor regulating body water homeostasis
- PMID: 19805236
- PMCID: PMC2747226
- DOI: 10.1073/pnas.0903695106
Secretin as a neurohypophysial factor regulating body water homeostasis
Abstract
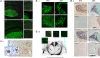
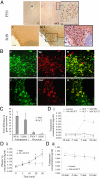
Hypothalamic magnocellular neurons express either one of the neurohypophysial hormones, vasopressin or oxytocin, along with different neuropeptides or neuromodulators. Axonal terminals of these neurons are generally accepted to release solely the two hormones but not others into the circulation. Here, we show that secretin, originally isolated from upper intestinal mucosal extract, is present throughout the hypothalamo-neurohypophysial axis and that it is released from the posterior pituitary under plasma hyperosmolality conditions. In the hypothalamus, it stimulates vasopressin expression and release. Considering these findings together with our previous findings that show a direct effect of secretin on renal water reabsorption, we propose here that secretin works at multiple levels in the hypothalamus, pituitary, and kidney to regulate water homeostasis. Findings presented here challenge previous understanding regarding the neurohypophysis and could provide new concepts in treating disorders related to osmoregulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
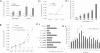
References
-
- Jeon US, et al. Oxytocin induces apical and basolateral redistribution of aquaporin-2 in rat kidney. Nephron Exp Nephrol. 2003;93:e36–e45. - PubMed
-
- Villanger O, Veel T, Raeder MG. Secretin causes H+/HCO3− secretion from pig pancreatic ductules by vacuolar-type H+-adenosine triphosphatase. Gastroenterology. 1995;108:850–859. - PubMed
-
- Marinelli RA, Pham L, Agre P, LaRusso NF. Secretin promotes osmotic water transport in rat cholangiocytes by increasing aquaporin-1 water channels in plasma membrane. Evidence for a secretin-induced vesicular translocation of aquaporin-1. J Biol Chem. 1997;272:12984–12988. - PubMed
-
- Tietz PS, et al. Agonist-induced coordinated trafficking of functionally related transport proteins for water and ions in cholangiocytes. J Biol Chem. 2003;278:20413–20419. - PubMed
-
- Chow BK, et al. Secretin controls anion secretion in the rat epididymis in an autocrine/paracrine fashion. Biol Reprod. 2004;70:1594–1599. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

