NO formation by a catalytically self-sufficient bacterial nitric oxide synthase from Sorangium cellulosum
- PMID: 19805284
- PMCID: PMC2752531
- DOI: 10.1073/pnas.0908443106
NO formation by a catalytically self-sufficient bacterial nitric oxide synthase from Sorangium cellulosum
Abstract
The role of nitric oxide (NO) in the host response to infection and in cellular signaling is well established. Enzymatic synthesis of NO is catalyzed by the nitric oxide synthases (NOSs), which convert Arg into NO and citrulline using co-substrates O2 and NADPH. Mammalian NOS contains a flavin reductase domain (FAD and FMN) and a catalytic heme oxygenase domain (P450-type heme and tetrahydrobiopterin). Bacterial NOSs, while much less studied, were previously identified as only containing the heme oxygenase domain of the more complex mammalian NOSs. We report here on the characterization of a NOS from Sorangium cellulosum (both full-length, scNOS, and oxygenase domain, scNOSox). scNOS contains a catalytic, oxygenase domain similar to those found in the mammalian NOS and in other bacteria. Unlike the other bacterial NOSs reported to date, however, this protein contains a fused reductase domain. The scNOS reductase domain is unique for the entire NOS family because it utilizes a 2Fe2S cluster for electron transfer. scNOS catalytically produces NO and citrulline in the presence of either tetrahydrobiopterin or tetrahydrofolate. These results establish a bacterial electron transfer pathway used for biological NO synthesis as well as a unique flexibility in using different tetrahydropterin cofactors for this reaction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
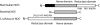

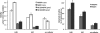
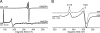

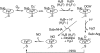
References
-
- Dinerman JL, Lowenstein CJ, Snyder SH. Molecular mechanisms of nitric oxide regulation—Potential relevance to cardiovascular disease. Circ Res. 1993;73:217–222. - PubMed
-
- Kerwin JF, Lancaster JR, Feldman PL. Nitric oxide—A new paradigm for 2nd messengers. J Med Chem. 1995;38:4343–4362. - PubMed
-
- Moncada S, Palmer RMJ, Higgs EA. Nitric oxide—Physiology, pathophysiology and pharmacology. Pharmacol Rev. 1991;43:109–142. - PubMed
-
- Li HY, Poulos TL. Structure-function studies on nitric oxide synthases. J Inorg Biochem. 2005;99:293–305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

