A polycomb repressive complex 2 gene regulates apogamy and gives evolutionary insights into early land plant evolution
- PMID: 19805300
- PMCID: PMC2752547
- DOI: 10.1073/pnas.0906997106
A polycomb repressive complex 2 gene regulates apogamy and gives evolutionary insights into early land plant evolution
Abstract
Land plants have distinct developmental programs in haploid (gametophyte) and diploid (sporophyte) generations. Although usually the two programs strictly alternate at fertilization and meiosis, one program can be induced during the other program. In a process called apogamy, cells of the gametophyte other than the egg cell initiate sporophyte development. Here, we report for the moss Physcomitrella patens that apogamy resulted from deletion of the gene orthologous to the Arabidopsis thaliana CURLY LEAF (PpCLF), which encodes a component of polycomb repressive complex 2 (PRC2). In the deletion lines, a gametophytic vegetative cell frequently gave rise to a sporophyte-like body. This body grew indeterminately from an apical cell with the character of a sporophytic pluripotent stem cell but did not form a sporangium. Furthermore, with continued culture, the sporophyte-like body branched. Sporophyte branching is almost unknown among extant bryophytes. When PpCLF was expressed in the deletion lines once the sporophyte-like bodies had formed, pluripotent stem cell activity was arrested and a sporangium-like organ formed. Supported by the observed pattern of PpCLF expression, these results demonstrate that, in the gametophyte, PpCLF represses initiation of a sporophytic pluripotent stem cell and, in the sporophyte, represses that stem cell activity and induces reproductive organ development. In land plants, branching, along with indeterminate apical growth and delayed initiation of spore-bearing reproductive organs, were conspicuous innovations for the evolution of a dominant sporophyte plant body. Our study provides insights into the role of PRC2 gene regulation for sustaining evolutionary innovation in land plants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
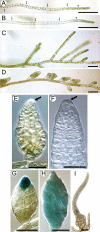

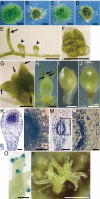
References
-
- Koltunow AM, Grossniklaus U. Apomixis: A developmental perspective. Annu Rev Plant Biol. 2003;54:547–574. - PubMed
-
- Asker SE, Jerling L. Apomixis in Plants. Boca Raton, FL: CRC Press; 1992.
-
- Yang H-Y, Zhou C. Experimental plant reproductive biology and reproductive cell manipulation in higher plants: Now and the future. Am J Bot. 1992;79:354–363.
-
- Gifford EM, Foster AS. Morphology and Evolution of Vascular Plants. 3rd Ed. Freeman and Co.: New York; 1989.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

