Genetic perturbation of postsynaptic activity regulates synapse elimination in developing cerebellum
- PMID: 19805323
- PMCID: PMC2752512
- DOI: 10.1073/pnas.0907298106
Genetic perturbation of postsynaptic activity regulates synapse elimination in developing cerebellum
Abstract
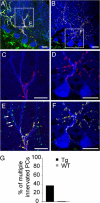
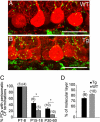
In many parts of the vertebrate nervous system, synaptic connections are remodeled during early postnatal life. Neural activity plays an important role in regulating one such rearrangement, synapse elimination, in the developing neuromuscular system, but there is little direct evidence on roles of pre- or postsynaptic activity in regulating synapse elimination in the developing brain. To address this issue, we expressed a chloride channel-yellow fluorescent protein fusion in cerebellar Purkinje cells (PCs) of transgenic mice to decrease their excitability. We then assessed elimination of supernumerary climbing fiber inputs to PCs. Individual PCs are innervated by multiple climbing fibers at birth; all but one are eliminated during the first three postnatal weeks in wild-type mice, but multiple innervation persists for at least three months in the transgenic mice. The normal redistribution of climbing fiber synapses from PC somata to proximal dendrites was also blunted in transgenics. These results show that normal electrical activity of the postsynaptic cell is required for it to attain a mature innervation pattern.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. - PubMed
-
- Buffelli M, Busetto G, Bidoia C, Favero M, Cangiano A. Activity-dependent synaptic competition at mammalian neuromuscular junctions. News Physiol Sci. 2004;19:85–91. - PubMed
-
- Lichtman JW, Colman H. Synapse elimination and indelible memory. Neuron. 2000;25:269–278. - PubMed
-
- Buffelli M, et al. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424:430–434. - PubMed
-
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

