No facilitator required for membrane transport of hydrogen sulfide
- PMID: 19805349
- PMCID: PMC2757810
- DOI: 10.1073/pnas.0902952106
No facilitator required for membrane transport of hydrogen sulfide
Abstract
Hydrogen sulfide (H(2)S) has emerged as a new and important member in the group of gaseous signaling molecules. However, the molecular transport mechanism has not yet been identified. Because of structural similarities with H(2)O, it was hypothesized that aquaporins may facilitate H(2)S transport across cell membranes. We tested this hypothesis by reconstituting the archeal aquaporin AfAQP from sulfide reducing bacteria Archaeoglobus fulgidus into planar membranes and by monitoring the resulting facilitation of osmotic water flow and H(2)S flux. To measure H(2)O and H(2)S fluxes, respectively, sodium ion dilution and buffer acidification by proton release (H(2)S left arrow over right arrow H(+) + HS(-)) were recorded in the immediate membrane vicinity. Both sodium ion concentration and pH were measured by scanning ion-selective microelectrodes. A lower limit of lipid bilayer permeability to H(2)S, P(M,H(2)S) >or = 0.5 +/- 0.4 cm/s was calculated by numerically solving the complete system of differential reaction diffusion equations and fitting the theoretical pH distribution to experimental pH profiles. Even though reconstitution of AfAQP significantly increased water permeability through planar lipid bilayers, P(M,H(2)S) remained unchanged. These results indicate that lipid membranes may well act as a barrier to water transport although they do not oppose a significant resistance to H(2)S diffusion. The fact that cholesterol and sphingomyelin reconstitution did not turn these membranes into an H(2)S barrier indicates that H(2)S transport through epithelial barriers, endothelial barriers, and membrane rafts also occurs by simple diffusion and does not require facilitation by membrane channels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
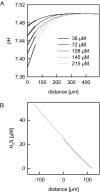




References
-
- Pryor WA, et al. Free radical biology and medicine: It's a gas, man! Am J Physiol Regul Integr Comp Physiol. 2006;291:R491–R511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

