Dual role of K ATP channel C-terminal motif in membrane targeting and metabolic regulation
- PMID: 19805355
- PMCID: PMC2757796
- DOI: 10.1073/pnas.0907138106
Dual role of K ATP channel C-terminal motif in membrane targeting and metabolic regulation
Abstract
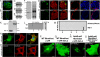
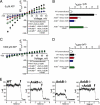
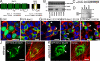
The coordinated sorting of ion channels to specific plasma membrane domains is necessary for excitable cell physiology. K(ATP) channels, assembled from pore-forming (Kir6.x) and regulatory sulfonylurea receptor subunits, are critical electrical transducers of the metabolic state of excitable tissues, including skeletal and smooth muscle, heart, brain, kidney, and pancreas. Here we show that the C-terminal domain of Kir6.2 contains a motif conferring membrane targeting in primary excitable cells. Kir6.2 lacking this motif displays aberrant channel targeting due to loss of association with the membrane adapter ankyrin-B (AnkB). Moreover, we demonstrate that this Kir6.2 C-terminal AnkB-binding motif (ABM) serves a dual role in K(ATP) channel trafficking and membrane metabolic regulation and dysfunction in these pathways results in human excitable cell disease. Thus, the K(ATP) channel ABM serves as a previously unrecognized bifunctional touch-point for grading K(ATP) channel gating and membrane targeting and may play a fundamental role in controlling excitable cell metabolic regulation.
Figures


References
-
- Seino S. ATP-sensitive potassium channels: A model of heteromultimeric potassium channel/receptor assemblies. Annu Rev Physiol. 1999;61:337–362. - PubMed
-
- Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. - PubMed
-
- Gloyn AL, Siddiqui J, Ellard S. Mutations in the genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum Mutat. 2006;27:220–231. - PubMed
-
- Bahi-Buisson N, et al. Infantile spasms as an epileptic feature of DEND syndrome associated with an activating mutation in the potassium adenosine triphosphate (ATP) channel, Kir6.2. J Child Neurol. 2007;22:1147–1150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL084583/HL/NHLBI NIH HHS/United States
- R01 HL079031/HL/NHLBI NIH HHS/United States
- HL70250/HL/NHLBI NIH HHS/United States
- R01 HL096652/HL/NHLBI NIH HHS/United States
- HL092232/HL/NHLBI NIH HHS/United States
- R01 HL070250/HL/NHLBI NIH HHS/United States
- R01 HL095010/HL/NHLBI NIH HHS/United States
- HL53268/HL/NHLBI NIH HHS/United States
- HL62494/HL/NHLBI NIH HHS/United States
- R01 HL062494/HL/NHLBI NIH HHS/United States
- R00 HL092232/HL/NHLBI NIH HHS/United States
- K99 HL092232/HL/NHLBI NIH HHS/United States
- R01 HL083422/HL/NHLBI NIH HHS/United States
- HL083422/HL/NHLBI NIH HHS/United States
- HL079031/HL/NHLBI NIH HHS/United States
- R01 HL084583/HL/NHLBI NIH HHS/United States
- HL95010/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

