HIF-2alpha maintains an undifferentiated state in neural crest-like human neuroblastoma tumor-initiating cells
- PMID: 19805377
- PMCID: PMC2745331
- DOI: 10.1073/pnas.0904606106
HIF-2alpha maintains an undifferentiated state in neural crest-like human neuroblastoma tumor-initiating cells
Abstract
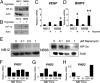
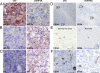
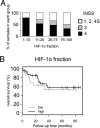
High hypoxia-inducible factor-2alpha (HIF-2alpha) protein levels predict poor outcome in neuroblastoma, and hypoxia dedifferentiates cultured neuroblastoma cells toward a neural crest-like phenotype. Here, we identify HIF-2alpha as a marker of normoxic neural crest-like neuroblastoma tumor-initiating/stem cells (TICs) isolated from patient bone marrows. Knockdown of HIF-2alpha reduced VEGF expression and induced partial sympathetic neuronal differentiation when these TICs were grown in vitro under stem cell-promoting conditions. Xenograft tumors of HIF-2alpha-silenced cells were widely necrotic, poorly vascularized, and resembled the bulk of tumor cells in clinical neuroblastomas by expressing additional sympathetic neuronal markers, whereas control tumors were immature, well-vascularized, and stroma-rich. Thus, HIF-2alpha maintains an undifferentiated state of neuroblastoma TICs. Because low differentiation is associated with poor outcome and angiogenesis is crucial for tumor growth, HIF-2alpha is an attractive target for neuroblastoma therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
High levels of HIF-2alpha highlight an immature neural crest-like neuroblastoma cell cohort located in a perivascular niche.J Pathol. 2008 Mar;214(4):482-8. doi: 10.1002/path.2304. J Pathol. 2008. PMID: 18189331
-
HIF-2alpha expression in human fetal paraganglia and neuroblastoma: relation to sympathetic differentiation, glucose deficiency, and hypoxia.Exp Cell Res. 2005 Feb 15;303(2):447-56. doi: 10.1016/j.yexcr.2004.10.003. Exp Cell Res. 2005. PMID: 15652356
-
The HIF-2α-driven pseudo-hypoxic phenotype in tumor aggressiveness, differentiation, and vascularization.Curr Top Microbiol Immunol. 2010;345:1-20. doi: 10.1007/82_2010_72. Curr Top Microbiol Immunol. 2010. PMID: 20517717 Review.
-
Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype.Proc Natl Acad Sci U S A. 2002 May 14;99(10):7021-6. doi: 10.1073/pnas.102660199. Proc Natl Acad Sci U S A. 2002. PMID: 12011461 Free PMC article.
-
Hypoxia, pseudohypoxia and cellular differentiation.Exp Cell Res. 2017 Jul 15;356(2):192-196. doi: 10.1016/j.yexcr.2017.03.007. Epub 2017 Mar 8. Exp Cell Res. 2017. PMID: 28284840 Review.
Cited by
-
Allosteric inhibition of hypoxia inducible factor-2 with small molecules.Nat Chem Biol. 2013 Apr;9(4):271-6. doi: 10.1038/nchembio.1185. Epub 2013 Feb 24. Nat Chem Biol. 2013. PMID: 23434853 Free PMC article.
-
Inhibition of hypoxia inducible factors combined with all-trans retinoic acid treatment enhances glial transdifferentiation of neuroblastoma cells.Sci Rep. 2015 Jun 9;5:11158. doi: 10.1038/srep11158. Sci Rep. 2015. PMID: 26057707 Free PMC article.
-
Hypoxia inducible factor pathway inhibitors as anticancer therapeutics.Future Med Chem. 2013 Apr;5(5):553-72. doi: 10.4155/fmc.13.17. Future Med Chem. 2013. PMID: 23573973 Free PMC article. Review.
-
Hypoxia in the Initiation and Progression of Neuroblastoma Tumours.Int J Mol Sci. 2019 Dec 19;21(1):39. doi: 10.3390/ijms21010039. Int J Mol Sci. 2019. PMID: 31861671 Free PMC article. Review.
-
Hypoxia-inducible factors and the response to hypoxic stress.Mol Cell. 2010 Oct 22;40(2):294-309. doi: 10.1016/j.molcel.2010.09.022. Mol Cell. 2010. PMID: 20965423 Free PMC article. Review.
References
-
- Pietras A, et al. High levels of HIF-2alpha highlight an immature neural crest-like neuroblastoma cell cohort located in a perivascular niche. J Pathol. 2008;214:482–488. - PubMed
-
- Howard MJ. Mechanisms and perspectives on differentiation of autonomic neurons. Dev Biol. 2005;277:271–286. - PubMed
-
- Helczynska K, et al. Hypoxia-inducible factor-2alpha correlates to distant recurrence and poor outcome in invasive breast cancer. Cancer Res. 2008;68:9212–9220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

