Transcription factor Glis3, a novel critical player in the regulation of pancreatic beta-cell development and insulin gene expression
- PMID: 19805515
- PMCID: PMC2786880
- DOI: 10.1128/MCB.01259-09
Transcription factor Glis3, a novel critical player in the regulation of pancreatic beta-cell development and insulin gene expression
Erratum in
- Mol Cell Biol. 2010 Apr;30(7):1864. Pierreux, Christophe E [added]; Lemaigre, Frederic P [added]
Abstract
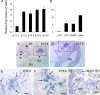
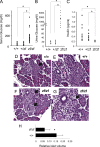
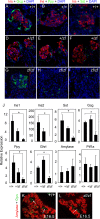
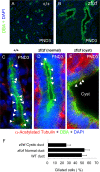
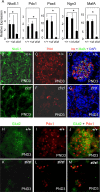
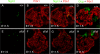
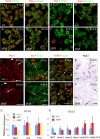
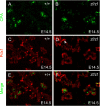
In this study, we report that the Krüppel-like zinc finger transcription factor Gli-similar 3 (Glis3) is induced during the secondary transition of pancreatic development, a stage of cell lineage specification and extensive patterning, and that Glis3(zf/zf) mutant mice develop neonatal diabetes, evidenced by hyperglycemia and hypoinsulinemia. The Glis3(zf/zf) mutant mouse pancreas shows a dramatic loss of beta and delta cells, contrasting a smaller relative loss of alpha, PP, and epsilon cells. In addition, Glis3(zf/zf) mutant mice develop ductal cysts, while no significant changes were observed in acini. Gene expression profiling and immunofluorescent staining demonstrated that the expression of pancreatic hormones and several transcription factors important in endocrine cell development, including Ngn3, MafA, and Pdx1, were significantly decreased in the developing pancreata of Glis3(zf/zf) mutant mice. The population of pancreatic progenitors appears not to be greatly affected in Glis3(zf/zf) mutant mice; however, the number of neurogenin 3 (Ngn3)-positive endocrine cell progenitors is significantly reduced. Our study indicates that Glis3 plays a key role in cell lineage specification, particularly in the development of mature pancreatic beta cells. In addition, we provide evidence that Glis3 regulates insulin gene expression through two Glis-binding sites in its proximal promoter, indicating that Glis3 also regulates beta-cell function.
Figures


References
-
- Ackermann, A. M., and M. Gannon. 2007. Molecular regulation of pancreatic beta-cell mass development, maintenance, and expansion. J. Mol. Endocrinol. 38:193-206. - PubMed
-
- Ahlgren, U., S. L. Pfaff, T. M. Jessell, T. Edlund, and H. Edlund. 1997. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature 385:257-260. - PubMed
-
- Andrali, S. S., M. L. Sampley, N. L. Vanderford, and S. Ozcan. 2008. Glucose regulation of insulin gene expression in pancreatic beta-cells. Biochem. J. 415:1-10. - PubMed
-
- Apelqvist, A., H. Li, L. Sommer, P. Beatus, D. J. Anderson, T. Honjo, M. Hrabe de Angelis, U. Lendahl, and H. Edlund. 1999. Notch signalling controls pancreatic cell differentiation. Nature 400:877-881. - PubMed
-
- Aramata, S., S. I. Han, and K. Kataoka. 2007. Roles and regulation of transcription factor MafA in islet beta-cells. Endocr. J. 54:659-666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
