The Sca2 autotransporter protein from Rickettsia conorii is sufficient to mediate adherence to and invasion of cultured mammalian cells
- PMID: 19805531
- PMCID: PMC2786473
- DOI: 10.1128/IAI.00201-09
The Sca2 autotransporter protein from Rickettsia conorii is sufficient to mediate adherence to and invasion of cultured mammalian cells
Abstract
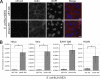
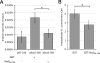
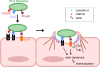
Obligate intracellular bacteria of the genus Rickettsia must adhere to and invade the host endothelium in order to establish an infection. These processes require the interaction of rickettsial surface proteins with mammalian host cell receptors. A previous bioinformatic analysis of sequenced rickettsial species identified a family of at least 17 predicted "surface cell antigen" (sca) genes whose products resemble autotransporter proteins. Two members of this family, rOmpA and rOmpB of spotted fever group (SFG) rickettsiae have been identified as adhesion and invasion factors, respectively; however, little is known about the putative functions of the other sca gene products. An intact sca2 gene is found in the majority of pathogenic SFG rickettsiae and, due to its sequence conservation among these species, we predict that Sca2 may play an important function at the rickettsial surface. Here we have shown that sca2 is transcribed and expressed in Rickettsia conorii and have used a heterologous gain-of-function assay in E. coli to determine the putative role of Sca2. Using this system, we have demonstrated that expression of Sca2 at the outer membrane of nonadherent, noninvasive E. coli is sufficient to mediate adherence to and invasion of a panel of mammalian cells, including endothelial cells. Furthermore, soluble Sca2 protein is capable of diminishing R. conorii invasion of cultured mammalian cells. This is the first evidence that Sca2 participates in the interaction between SFG rickettsiae and host cells and suggests that in addition to other surface proteins, Sca2 may play a critical role in rickettsial pathogenesis.
Figures


References
-
- Blanc, G., M. Ngwamidiba, H. Ogata, P. E. Fournier, J. M. Claverie, and D. Raoult. 2005. Molecular evolution of Rickettsia surface antigens: evidence of positive selection. Mol. Biol. Evol. 20:2073-2083. - PubMed
-
- Cossart, P., and P. J. Sansonetti. 2004. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304:242-248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

