Bax activates endophilin B1 oligomerization and lipid membrane vesiculation
- PMID: 19805544
- PMCID: PMC2797207
- DOI: 10.1074/jbc.M109.021873
Bax activates endophilin B1 oligomerization and lipid membrane vesiculation
Abstract
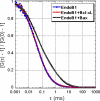
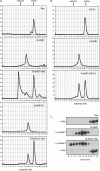
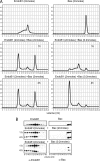
Endophilins participate in membrane scission events that occur during endocytosis and intracellular organelle biogenesis through the combined activity of an N-terminal BAR domain that interacts with membranes and a C-terminal SH3 domain that mediates protein binding. Endophilin B1 (Endo B1) was identified to bind Bax, a Bcl-2 family member that promotes apoptosis, through yeast two-hybrid protein screens. Although Endo B1 does not bind Bax in healthy cells, during apoptosis, Endo B1 interacts transiently with Bax and promotes cytochrome c release from mitochondria. To explore the molecular mechanism of action of Endo B1, we have analyzed its interaction with Bax in cell-free systems. Purified recombinant Endo B1 in solution displays a Stokes radius indicating a tetrameric quarternary structure. However, when incubated with purified Bax, it assembles into oligomers more than 4-fold greater in molecular weight. Although Endo B1 oligomerization is induced by Bax, Bax does not stably associate with the high molecular weight Endo B1 complex. Endo B1 oligomerization requires its C-terminal Src homology 3 domain and is not induced by Bcl-xL. Endo B1 combined with Bax reduces the size and changes the morphology of giant unilamellar vesicles by inducing massive vesiculation of liposomes. This activity of purified Bax protein to induce cell-free assembly of Endo B1 may reflect its activity in cells that regulates apoptosis and/or mitochondrial fusion.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

