Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial
- PMID: 19805702
- PMCID: PMC2878137
- DOI: 10.1001/archgenpsychiatry.2009.128
Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial
Abstract
Context: Cocaine dependence, which affects 2.5 million Americans annually, has no US Food and Drug Administration-approved pharmacotherapy.
Objectives: To evaluate the immunogenicity, safety, and efficacy of a novel cocaine vaccine to treat cocaine dependence.
Design: A 24-week, phase 2b, randomized, double-blind, placebo-controlled trial with efficacy assessed during weeks 8 to 20 and follow-up to week 24.
Setting: Cocaine- and opioid-dependent persons recruited from October 2003 to April 2005 from greater New Haven, Connecticut.
Participants: One hundred fifteen methadone-maintained subjects (67% male, 87% white, aged 18-46 years) were randomized to vaccine or placebo, and 94 subjects (82%) completed the trial. Most smoked crack cocaine along with using marijuana (18%), alcohol (10%), and nonprescription opioids (44%).
Intervention: Over 12 weeks, 109 of 115 subjects received 5 vaccinations of placebo or succinylnorcocaine linked to recombinant cholera toxin B-subunit protein. Main Outcome Measure Semiquantitative urinary cocaine metabolite levels measured thrice weekly with a positive cutoff of 300 ng/mL.
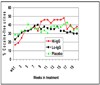
Results: The 21 vaccinated subjects (38%) who attained serum IgG anticocaine antibody levels of 43 microg/mL or higher (ie, high IgG level) had significantly more cocaine-free urine samples than those with levels less than 43 microg/mL (ie, low IgG level) and the placebo-receiving subjects during weeks 9 to 16 (45% vs 35% cocaine-free urine samples, respectively). The proportion of subjects having a 50% reduction in cocaine use was significantly greater in the subjects with a high IgG level than in subjects with a low IgG level (53% of subjects vs 23% of subjects, respectively) (P = .048). The most common adverse effects were injection site induration and tenderness. There were no treatment-related serious adverse events, withdrawals, or deaths.
Conclusions: Attaining high (>or=43 microg/mL) IgG anticocaine antibody levels was associated with significantly reduced cocaine use, but only 38% of the vaccinated subjects attained these IgG levels and they had only 2 months of adequate cocaine blockade. Thus, we need improved vaccines and boosters. Trial Registration clinicaltrials.gov Identifier: NCT00142857.
Figures



References
-
- McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. - PubMed
-
- National Estimates of Drug-related Emergency Department Visits. Substance Abuse and Mental Health Services Administration: Year-End 2005. 2005. http://dawninfo.samhsa.gov. . Updated Last Updated Date.
-
- Substance Abuse and Mental Health Services Administration (SAMHSA) Summary of Findings from the 2007 National Household Survey on Drug Abuse. Rockville Maryland: Department of Health and Human Services, Substance Abuse and Mental Health Services Administration; 2008.
-
- Silva de Lima M, Garcia de Oliveira Soares G, Alves Pereira Reisser A, Farrell M. Pharmacological treatment of cocaine dependence: a systematic review. Addiction. 2002;97(8):931–949. - PubMed
-
- Vocci FJ, Jr, Elkashef A. Pharamcotherapy and other treatments for cocaine abuse and dependence. Curr Opinion Psychiatry. 2005;18:265–270. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

