Cell-cell and intracellular lactate shuttles
- PMID: 19805739
- PMCID: PMC2805372
- DOI: 10.1113/jphysiol.2009.178350
Cell-cell and intracellular lactate shuttles
Abstract
Once thought to be the consequence of oxygen lack in contracting skeletal muscle, the glycolytic product lactate is formed and utilized continuously in diverse cells under fully aerobic conditions. 'Cell-cell' and 'intracellular lactate shuttle' concepts describe the roles of lactate in delivery of oxidative and gluconeogenic substrates as well as in cell signalling. Examples of the cell-cell shuttles include lactate exchanges between between white-glycolytic and red-oxidative fibres within a working muscle bed, and between working skeletal muscle and heart, brain, liver and kidneys. Examples of intracellular lactate shuttles include lactate uptake by mitochondria and pyruvate for lactate exchange in peroxisomes. Lactate for pyruvate exchanges affect cell redox state, and by itself lactate is a ROS generator. In vivo, lactate is a preferred substrate and high blood lactate levels down-regulate the use of glucose and free fatty acids (FFA). As well, lactate binding may affect metabolic regulation, for instance binding to G-protein receptors in adipocytes inhibiting lipolysis, and thus decreasing plasma FFA availability. In vitro lactate accumulation upregulates expression of MCT1 and genes coding for other components of the mitochondrial reticulum in skeletal muscle. The mitochondrial reticulum in muscle and mitochondrial networks in other aerobic tissues function to establish concentration and proton gradients necessary for cells with high mitochondrial densities to oxidize lactate. The presence of lactate shuttles gives rise to the realization that glycolytic and oxidative pathways should be viewed as linked, as opposed to alternative, processes, because lactate, the product of one pathway, is the substrate for the other.
Figures
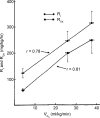
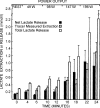
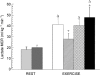
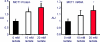
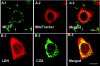

References
-
- Bergman BC, Horning MA, Casazza GA, Wolfel EE, Butterfield GE, Brooks GA. Endurance training increases gluconeogenesis during rest and exercise in men. Am J Physiol Endocrinol Metab. 2000;278:E244–251. - PubMed
-
- Bergman BC, Wolfel EE, Butterfield GE, Lopaschuk G, Casazza GA, Horning MA, Brooks GA. Active muscle and whole body lactate kinetics after endurance training in men. J Appl Physiol. 1999;87:1684–1696. - PubMed
-
- Bertocci LA, Lujan BF. Oxidative incorporation and utilization of [3-13C]lactate and [1,2-13C]acetate by rat skeletal muscle. J Appl Physiol. 1999;86:2077–2089. - PubMed
-
- Brandt RB, Laux JE, Spainhour SE, Kline ES. Lactate dehydrogenase in mitochondria. Arch Biochem Biophys. 1987;259:412–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

