Adoptive immunotherapy with liver allograft-derived lymphocytes induces anti-HCV activity after liver transplantation in humans and humanized mice
- PMID: 19805910
- PMCID: PMC2769186
- DOI: 10.1172/JCI38374
Adoptive immunotherapy with liver allograft-derived lymphocytes induces anti-HCV activity after liver transplantation in humans and humanized mice
Abstract
After liver transplantation in HCV-infected patients, the virus load inevitably exceeds pre-transplantation levels. This phenomenon reflects suppression of the host-effector immune responses that control HCV replication by the immunosuppressive drugs used to prevent rejection of the transplanted liver. Here, we describe an adoptive immunotherapy approach, using lymphocytes extracted from liver allograft perfusate (termed herein liver allograft-derived lymphocytes), which includes an abundance of NK/NKT cells that mounted an anti-HCV response in HCV-infected liver transplantation recipients, despite the immunosuppressive environment. This therapy involved intravenously injecting patients 3 days after liver transplantation with liver allograft-derived lymphocytes treated with IL-2 and the CD3-specific mAb OKT3. During the first month after liver transplantation, the HCV RNA titers in the sera of recipients who received immunotherapy were markedly lower than those in the sera of recipients who did not receive immunotherapy. We further explored these observations in human hepatocyte-chimeric mice, in which mouse hepatocytes were replaced by human hepatocytes. These mice unfailingly developed HCV infections after inoculation with HCV-infected human serum. However, injection of human liver-derived lymphocytes treated with IL-2/OKT3 completely prevented HCV infection. Furthermore, an in vitro study using genomic HCV replicon-containing hepatic cells revealed that IFN-gamma-secreting cells played a pivotal role in such anti-HCV responses. Thus, our study presents what we believe to be a novel paradigm for the inhibition of HCV replication in HCV-infected liver transplantation recipients.
Figures
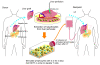


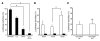

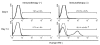
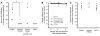
Comment in
-
Preventing hepatitis C virus recurrence in liver transplant recipients: a role for adoptive immunotherapy?Hepatology. 2010 Mar;51(3):1072-6. doi: 10.1002/hep.23579. Hepatology. 2010. PMID: 20198701 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

