Suppression of KATP channel activity protects murine pancreatic beta cells against oxidative stress
- PMID: 19805912
- PMCID: PMC2769178
- DOI: 10.1172/JCI38817
Suppression of KATP channel activity protects murine pancreatic beta cells against oxidative stress
Abstract
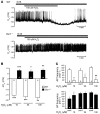
The enhanced oxidative stress associated with type 2 diabetes mellitus contributes to disease pathogenesis. We previously identified plasma membrane-associated ATP-sensitive K+ (KATP) channels of pancreatic beta cells as targets for oxidants. Here, we examined the effects of genetic and pharmacologic ablation of KATP channels on loss of mouse beta cell function and viability following oxidative stress. Using mice lacking the sulfonylurea receptor type 1 (Sur1) subunit of KATP channels, we found that, compared with insulin secretion by WT islets, insulin secretion by Sur1-/- islets was less susceptible to oxidative stress induced by the oxidant H2O2. This was likely, at least in part, a result of the reduced ability of H2O2 to hyperpolarize plasma membrane potential and reduce cytosolic free Ca2+ concentration ([Ca2+]c) in the Sur1-/- beta cells. Remarkably, Sur1-/- beta cells were less prone to apoptosis induced by H2O2 or an NO donor than WT beta cells, despite an enhanced basal rate of apoptosis. This protective effect was attributed to upregulation of the antioxidant enzymes SOD, glutathione peroxidase, and catalase. Upregulation of antioxidant enzymes and reduced sensitivity of Sur1-/- cells to H2O2-induced apoptosis were mimicked by treatment with the sulfonylureas tolbutamide and gliclazide. Enzyme upregulation and protection against oxidant-induced apoptosis were abrogated by agents lowering [Ca2+]c. Sur1-/- mice were less susceptible than WT mice to streptozotocin-induced beta cell destruction and subsequent hyperglycemia and death, which suggests that loss of KATP channel activity may protect against streptozotocin-induced diabetes in vivo.
Figures









References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

