Stabilization of Ca current in Purkinje neurons during high-frequency firing by a balance of Ca-dependent facilitation and inactivation
- PMID: 19806011
- PMCID: PMC2897944
- DOI: 10.4161/chan.3.6.9838
Stabilization of Ca current in Purkinje neurons during high-frequency firing by a balance of Ca-dependent facilitation and inactivation
Abstract
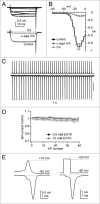
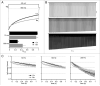
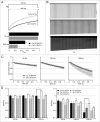
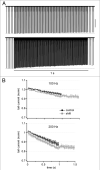
Purkinje neurons fire spontaneous action potentials at ∼50 spikes/sec and generate more than 100 spikes/sec during cerebellum-mediated behaviors. Many voltage-gated channels, including Ca channels, can inactivate and/or facilitate with repeated stimulation, raising the question of how these channels respond to regular, rapid trains of depolarizations. To test whether Ca currents are modulated during firing, we recorded voltage-clamped Ca currents, predominantly carried by P-type Ca channels, from acutely dissociated mouse Purkinje neurons at 30-33°C (1 mM Ca). With 0.5 mM intracellular EGTA, 1-second trains of either spontaneous action potential waveforms or brief depolarizing steps at 50 Hz evoked Ca tail currents that were stable, remaining within 5% of the first tail current throughout the train. Higher frequency trains (100 and 200 Hz) elicited a maximal inactivation of <10%. To test whether this stability of Ca currents resulted from a lack of modulation or from an equilibrium between facilitation and inactivation, we manipulated the permeant ion (Ca vs. Ba) and Ca buffering (0.5 vs. 10 mM EGTA). With low buffering, Ba accelerated the initial inactivation evoked by 1-second trains, but reduced its extent at 200 Hz, consistent with an early calcium-dependent facilitation (CDF) and late calcium-dependent inactivation (CDI) at high frequencies. Increasing the Ca buffer favored CDF. These data suggest that stable Ca current amplitudes result from a balance of CDF, CDI, and voltage-dependent inactivation. This modest net Ca-dependent modulation may contribute to the ability of Purkinje neurons to sustain long periods of regular firing and synaptic transmission.
Figures

References
-
- Hausser M, Clark BA. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron. 1997;19:665–78. - PubMed
-
- Liang H, DeMaria CD, Erickson MG, Mori MX, Alseikhan BA, Yue DT. Unified mechanisms of Ca2+ regulation across the Ca2+ channel family. Neuron. 2003;39:951–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
