Multi-determinants analysis of molecular alterations for predicting clinical benefit to EGFR-targeted monoclonal antibodies in colorectal cancer
- PMID: 19806185
- PMCID: PMC2750753
- DOI: 10.1371/journal.pone.0007287
Multi-determinants analysis of molecular alterations for predicting clinical benefit to EGFR-targeted monoclonal antibodies in colorectal cancer
Abstract
Background: KRAS mutations occur in 35-45% of metastatic colorectal cancers (mCRC) and preclude responsiveness to EGFR-targeted therapy with cetuximab or panitumumab. However, less than 20% patients displaying wild-type KRAS tumors achieve objective response. Alterations in other effectors downstream of the EGFR, such as BRAF, and deregulation of the PIK3CA/PTEN pathway have independently been found to give rise to resistance. We present a comprehensive analysis of KRAS, BRAF, PIK3CA mutations, and PTEN expression in mCRC patients treated with cetuximab or panitumumab, with the aim of clarifying the relative contribution of these molecular alterations to resistance.
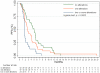
Methodology/principal findings: We retrospectively analyzed objective tumor response, progression-free (PFS) and overall survival (OS) together with the mutational status of KRAS, BRAF, PIK3CA and expression of PTEN in 132 tumors from cetuximab or panitumumab treated mCRC patients. Among the 106 non-responsive patients, 74 (70%) had tumors with at least one molecular alteration in the four markers. The probability of response was 51% (22/43) among patients with no alterations, 4% (2/47) among patients with 1 alteration, and 0% (0/24) for patients with > or =2 alterations (p<0.0001). Accordingly, PFS and OS were increasingly worse for patients with tumors harboring none, 1, or > or =2 molecular alteration(s) (p<0.001).
Conclusions/significance: When expression of PTEN and mutations of KRAS, BRAF and PIK3CA are concomitantly ascertained, up to 70% of mCRC patients unlikely to respond to anti-EGFR therapies can be identified. We propose to define as 'quadruple negative', the CRCs lacking alterations in KRAS, BRAF, PTEN and PIK3CA. Comprehensive molecular dissection of the EGFR signaling pathways should be considered to select mCRC patients for cetuximab- or panitumumab-based therapies.
Conflict of interest statement
Figures





References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Sargent DJ, Wieand HS, Haller DG, Gray R, Benedetti JK, et al. Disease-free survival versus overall survival as a primary endpoint for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23:8664–8670. - PubMed
-
- Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352:476–487. - PubMed
-
- Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. - PubMed
-
- Pessino A, Artale S, Sciallero S, Guglielmi A, Fornarini G, et al. First-line single-agent cetuximab in patients with advanced colorectal cancer. Ann Oncol. 2008;19:711–716. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

