Histopathology of type 1 diabetes: old paradigms and new insights
- PMID: 19806238
- PMCID: PMC2779010
- DOI: 10.1900/RDS.2009.6.85
Histopathology of type 1 diabetes: old paradigms and new insights
Abstract
Although our knowledge on the various aspects of diabetes development in the NOD mouse model is substantial and keeps expanding at a dramatic pace, the dataset on histopathologic features of type 1 diabetes (T1D) in patients remains largely stagnant. Early work has established an array of common aspects that have become epitomic in the absence of new patient material. There is a growing consensus that an updated and more detailed view is required that challenges and expands our understanding. Comprehensive initiatives are currently ongoing to address these issues in pre-diabetic, recent onset and longstanding type 1 diabetic individuals, and some of the old data have been recently revisited. In this review article, we wish to provide an overview of where we stand today and how we can correlate the various cross-sectional studies from the past with contemporary models of the disease. We believe an enhanced understanding of the many histopathological particularities in patients as compared to animal models will ultimately lead, not only to more fundamental insights, but also to an improved ability to translate pre-clinical data from bench to bedside.
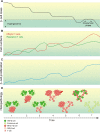
Figures

References
-
- Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965;14(10):619–633. - PubMed
-
- Bottazzo GF, Florin-Christensen A, Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974;2(7892):1279–1283. - PubMed
-
- MacCuish AC, Irvine WJ, Barnes EW, Duncan LJ. Antibodies to pancreatic islet cells in insulin-dependent diabetics with coexistent autoimmune disease. Lancet. 1974;2(7896):1529–1531. - PubMed
-
- Nerup J, Platz P, Andersen OO, Christy M, Lyngsoe J, Poulsen JE, Ryder LP, Nielsen LS, Thomsen M, Svejgaard A. HL-A antigens and diabetes mellitus. Lancet. 1974;2(7885):864–866. - PubMed
-
- Singal DP, Blajchman MA. Histocompatibility (HL-A) antigens, lymphocytotoxic antibodies and tissue antibodies in patients with diabetes mellitus. Diabetes. 1973;22:429–432. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
