Endogenous levels of Echinacea alkylamides and ketones are important contributors to the inhibition of prostaglandin E2 and nitric oxide production in cultured macrophages
- PMID: 19807154
- PMCID: PMC2777644
- DOI: 10.1021/jf901202y
Endogenous levels of Echinacea alkylamides and ketones are important contributors to the inhibition of prostaglandin E2 and nitric oxide production in cultured macrophages
Abstract
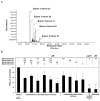
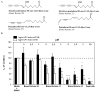
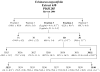
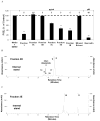
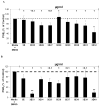
Because of the popularity of Echinacea as a dietary supplement, researchers have been actively investigating which Echinacea constituent or groups of constituents are necessary for immune-modulating bioactivities. Our prior studies indicate that alkylamides may play an important role in the inhibition of prostaglandin E2 (PGE(2)) production. High-performance liquid chromatography fractionation, employed to elucidate interacting anti-inflammatory constituents from ethanol extracts of Echinacea purpurea, Echinacea angustifolia, Echinacea pallida, and Echinacea tennesseensis, identified fractions containing alkylamides and ketones as key anti-inflammatory contributors using lipopolysaccharide-induced PGE(2) production in RAW264.7 mouse macrophage cells. Nitric oxide (NO) production and parallel cytotoxicity screens were also employed to substantiate an anti-inflammatory response. E. pallida showed significant inhibition of PGE(2) with a first round fraction, containing gas chromatography-mass spectrometry (GC-MS) peaks for Bauer ketones 20, 21, 22, 23, and 24, with 23 and 24 identified as significant contributors to this PGE(2) inhibition. Chemically synthesized Bauer ketones 21 and 23 at 1 microM each significantly inhibited both PGE(2) and NO production. Three rounds of fractionation were produced from an E. angustifolia extract. GC-MS analysis identified the presence of Bauer ketone 23 in third round fraction 3D32 and Bauer alkylamide 11 making up 96% of third round fraction 3E40. Synthetic Bauer ketone 23 inhibited PGE(2) production to 83% of control, and synthetic Bauer alkylamide 11 significantly inhibited PGE(2) and NO production at the endogenous concentrations determined to be present in their respective fraction; thus, each constituent partially explained the in vitro anti-inflammatory activity of their respective fraction. From this study, two key contributors to the anti-inflammatory properties of E. angustifolia were identified as Bauer alkylamide 11 and Bauer ketone 23.
Figures
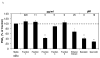
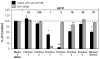
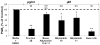
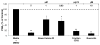
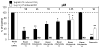
References
-
- Blumenthal M, Ferrier G, Cavaliere C. Total Sales of Herbal Supplements in United States Show Steady Growth. The Journal of the American Botanical Council. 2006;71:64–66.
-
- Chen Y, Fu T, Tao T, Yang J, Chang Y, Wang M, Kim L, Qu L, Cassady J, Scalzo R, Wang X. Macrophage activating effects of new alkamides from the roots of Echinacea species. J Nat Prod. 2005;68(5):773–6. - PubMed
-
- Clifford LJ, Nair MG, Rana J, Dewitt DL. Bioactivity of alkamides isolated from Echinacea purpurea (L.) Moench. Phytomedicine. 2002;9(3):249–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

