Gonad morphogenesis in vertebrates: divergent means to a convergent end
- PMID: 19807280
- PMCID: PMC4507502
- DOI: 10.1146/annurev.cellbio.042308.13350
Gonad morphogenesis in vertebrates: divergent means to a convergent end
Abstract
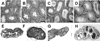
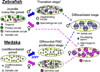
A critical element of successful sexual reproduction is the generation of sexually dimorphic adult reproductive organs, the testis and ovary, which produce functional gametes. Examination of different vertebrate species shows that the adult gonad is remarkably similar in its morphology across different phylogenetic classes. Surprisingly, however, the cellular and molecular programs employed to create similar organs are not evolutionarily conserved. We highlight the mechanisms used by different vertebrate model systems to generate the somatic architecture necessary to support gametogenesis. In addition, we examine the different vertebrate patterns of germ cell migration from their site of origin to colonize the gonad and highlight their roles in sex-specific morphogenesis. We also discuss the plasticity of the adult gonad and consider how different genetic and environmental conditions can induce transitions between testis and ovary morphology.
Figures





References
-
- Albrecht KH, Eicher EM. Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev. Biol. 2001;240:92–107. - PubMed
-
- Bauchinger U, Van’t Hof T, Biebach H. Testicular development during long-distance spring migration. Horm. Behav. 2007;51:295–305. - PubMed
-
- Bauchinger U, Wohlmann A, Biebach H. Flexible remodeling of organ size during spring migration of the garden warbler (Sylvia borin) Zoology (Jena) 2005;108:97–106. - PubMed
-
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, et al. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

