FRAGMATIC: a randomised phase III clinical trial investigating the effect of fragmin added to standard therapy in patients with lung cancer
- PMID: 19807917
- PMCID: PMC2761945
- DOI: 10.1186/1471-2407-9-355
FRAGMATIC: a randomised phase III clinical trial investigating the effect of fragmin added to standard therapy in patients with lung cancer
Abstract
Background: Venous thromboembolism (VTE) occurs when blood clots in the leg, pelvic or other deep vein (deep vein thrombosis) with or without transport of the thrombus into the pulmonary arterial circulation (pulmonary embolus). VTE is common in patients with cancer and is increased by surgery, chemotherapy, radiotherapy and disease progression. Low molecular weight heparin (LMWH) is routinely used to treat VTE and some evidence suggests that LMWH may also have an anticancer effect, by reduction in the incidence of metastases. The FRAGMATIC trial will assess the effect of adding dalteparin (FRAGMIN), a type of LMWH, to standard treatment for patients with lung cancer.
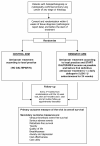
Methods/design: The study design is a randomised multicentre phase III trial comparing standard treatment and standard treatment plus daily LMWH for 24 weeks in patients with lung cancer. Patients eligible for this study must have histopathological or cytological diagnosis of primary bronchial carcinoma (small cell or non-small cell) within 6 weeks of randomisation, be 18 or older, and must be willing and able to self-administer 5000 IU dalteparin by daily subcutaneous injection or have it administered to themselves or by a carer for 24 weeks. A total of 2200 patients will be recruited from all over the UK over a 3 year period and followed up for a minimum of 1 year after randomisation. Patients will be randomised to one of the two treatment groups in a 1:1 ratio, standard treatment or standard treatment plus dalteparin. The primary outcome measure of the trial is overall survival. The secondary outcome measures include venous thrombotic event (VTE) free survival, serious adverse events (SAEs), metastasis-free survival, toxicity, quality of life (QoL), levels of breathlessness, anxiety and depression, cost effectiveness and cost utility.
Trial registration: Current Controlled Trials ISRCTN80812769.
References
-
- Quinn M, Babb P, Brock A, Kirby L, Jones J. Cancer trends in England and Wales. London, Stationary Office; 2001.
-
- Samet JM. The epidemiology of lung cancer. Chest. 1993;103:20–25. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical