MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2
- PMID: 19808020
- PMCID: PMC2759401
- DOI: 10.1016/j.cmet.2009.08.015
MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2
Abstract
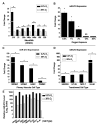
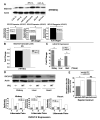
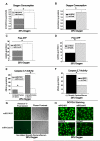
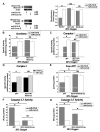
Repression of mitochondrial respiration represents an evolutionarily ancient cellular adaptation to hypoxia and profoundly influences cell survival and function; however, the underlying molecular mechanisms are incompletely understood. Primarily utilizing pulmonary arterial endothelial cells as a representative hypoxic cell type, we identify the iron-sulfur cluster assembly proteins (ISCU1/2) as direct targets for repression by the hypoxia-induced microRNA-210 (miR-210). ISCU1/2 facilitate the assembly of iron-sulfur clusters, prosthetic groups that are critical for electron transport and mitochondrial oxidation-reduction reactions. Under in vivo conditions of upregulating miR-210 and repressing ISCU1/2, the integrity of iron-sulfur clusters is disrupted. In turn, by repressing ISCU1/2 during hypoxia, miR-210 decreases the activity of prototypical iron-sulfur proteins controlling mitochondrial metabolism, including Complex I and aconitase. Consequently, miR-210 represses mitochondrial respiration and associated downstream functions. These results identify important mechanistic connections among microRNA, iron-sulfur cluster biology, hypoxia, and mitochondrial function, with broad implications for cellular metabolism and adaptation to cellular stress.
Figures


References
-
- Aisenberg AC, Potter VR. Studies on the Pasteur effect. II. Specific mechanisms. J Biol Chem. 1957;224:1115–1127. - PubMed
-
- Beinert H, Holm RH, Munck E. Iron-sulfur clusters: nature’s modular, multipurpose structures. Science. 1997;277:653–659. - PubMed
-
- Camps C, Buffa FM, Colella S, Moore J, Sotiriou C, Sheldon H, Harris AL, Gleadle JM, Ragoussis J. hsa-miR-210 Is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res. 2008;14:1340–1348. - PubMed
-
- Chavez A, Miranda LF, Pichiule P, Chavez JC. Mitochondria and hypoxia-induced gene expression mediated by hypoxia-inducible factors. Ann N Y Acad Sci. 2008;1147:312–320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

